HYDROCARBON PHASE BEHAVIOUR
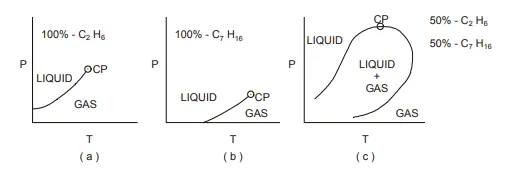
This subject has been covered extensively in specialist books8,13,18 and is described here in a somewhat perfunctory manner simply to provide a qualitative understanding of the difference between various hydrocarbon systems as they exist in the reservoir. Consider, first of all, the simple experiment in which a cylinder containing one of the lighter members of the paraffin series, C2 H6−ethane, is subjected to a continuously increasing pressure at constant temperature.
At some unique pressure (the vapour pressure) during this experiment the C2 H6, which was totally in the gas phase at low pressures will condense into a liquid. If this experiment were repeated at a series of different temperatures the resulting phase diagram, which is the pressure temperature relationship, could be drawn as shown in fig.

The line defining the pressures at which the transition from gas to liquid occurs, at different temperatures, is known as the vapour pressure line. It terminates at the critical point (CP) at which it is no longer possible to distinguish whether the fluid is liquid or gas, the intensive properties of both phases being identical. Above the vapour pressure line the fluid is entirely liquid while below it is in the gaseous state.
If the above experiment were repeated for a heavier member of the paraffin series, say, C7 H16 − heptane, the results would be as shown in fig. 1.14(b). One clear difference between (a) and (b) is that at lower temperatures and pressures there is a greater tendency for the heavier hydrocarbon, C7 H16, to be in the liquid state. For a two component system, the phase diagram for a 50% C2 H6 and 50% C7 H16 mixture would be as shown in fig. 1.14 (c).
In this case, while there are regions where the fluid mixture is either entirely gas or liquid, there is now also a clearly defined region in which the gas and liquid states can coexist; the, so-called, two phase region. The shape of the envelope defining the two phase region is dependent on the composition of the mixture, being more vertically inclined if the C2H6 is the predominant component and more horizontal if it is the C7 H16.
Naturally occurring hydrocarbons are more complex than the system shown in fig. 1.14 in that they contain a great many members of the paraffin series and usually some nonhydrocarbon impurities. Nevertheless, a phase diagram can similarly be defined for complex mixtures and such a diagram for a typical natural gas is shown in fig. 1.15(a).
The lines defining the two phase region are described as the bubble point line, separating the liquid from the two phase region, and the dew point line, separating the gas from the two phase region.
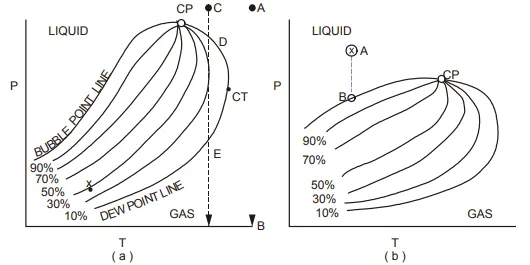
liquid to the two phase region, the first bubbles of gas will appear while, crossing the dew point line from the gas, the first drops of liquid (dew) will appear. The lines within the two phase region represent constant liquid saturations. For a gas field, as described in secs. 1.5 − 1.8, the reservoir temperature must be such that it exceeds the so-called cricondentherm (CT), which is the maximum temperature at which the two phases can coexist for the particular hydrocarbon mixture.
If the initial reservoir pressure and temperature are such that they coincide with point A in fig. 1.15(a), then for isothermal reservoir depletion, which is generally assumed, the pressure will decline from A towards point B and the dew point line will never be crossed. This means that only dry gas will exist in the reservoir at any pressure. On producing the gas to the surface, however, both pressure and temperature will decrease and the final state will be at some point X within the two phase envelope, the position of the point being dependent on the conditions of surface separation.
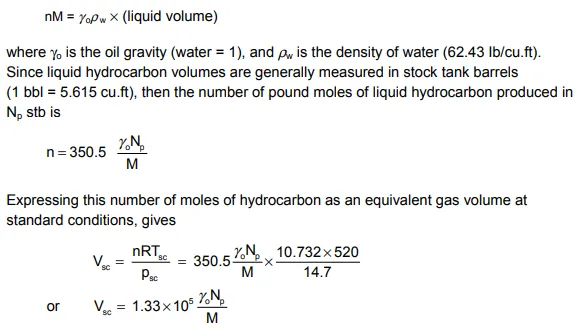
The material balance equations presented in this chapter, equs. (1.35) and (1.41), assumed that a volume of gas in the reservoir was produced as gas at the surface. If, due to surface separation, small amounts of liquid hydrocarbon are produced, the cumulative liquid volume must be converted into an equivalent gas volume and added to the cumulative gas production to give the correct value of Gp for use in the material balance equation.
Thus if n pound moles of liquid have been produced, of molecular weight M, then the total mass of liquid is
The maximum liquid saturation deposited in the reservoir, when the pressure is between points D and E in the two phase region, is generally rather small and frequently is below the critical saturation which must be exceeded before the liquid becomes mobile. This phenomenon is analogous to the residual saturations, discussed previously, at which flow ceases. Therefore, the liquid hydrocarbons deposited in the reservoir, which are referred to as retrograde liquid condensate, are not recovered and, since the heavier components tend to condense first, this represents a loss of the most valuable part of the hydrocarbon mixture. It may be imagined that continued pressure depletion below the dew point at E would lead to re-vapourisation of the liquid condensate. This does not occur, however, because once the pressure falls below point D the overall molecular weight of the hydrocarbons remaining in the reservoir increases, since some of the heavier paraffins are left behind in the reservoir as retrograde condensate. Therefore, the composite phase envelope for the reservoir fluids tends to move downwards and to the right thus inhibiting re-vapourisation.