
The Z-factor correlation of Standing and Katz
This correlation requires a knowledge of the composition of the gas or, at least, the gas gravity. Naturally occurring hydrocarbons are composed primarily of members of the paraffin series (CnH2n+2) with an admixture of non-hydrocarbon impurities such as carbon dioxide, nitrogen and hydrogen sulphide. Natural gas differs from oil in that it predominantly consists of the lighter members of the paraffin series, methane and ethane, which usually comprise in excess of 90% of the volume.
A typical gas composition is listed in table 1.1. In order to use the Standing-Katz correlation11 it is first necessary, from a knowledge of the gas composition, to determine the pseudo critical pressure and temperature of the mixture as

where the summation is over all the components present in the gas. The parameters pci and Tci are the critical pressure and temperature of the ith component, listed in table 1.1, while the ni are the volume fractions or, for a gas, the mole fractions of each component (Avogrado's law). The next step is to calculate the so-called pseudo reduced pressure and temperature

where p and T are the pressure and temperature at which it is required to determine Z. In the majority of reservoir engineering problems, which are isothermal, Tpr is constant and ppr variable. With these two parameters the Standing-Katz correlation chart, fig. 1.6, which consists of a set of isotherms giving Z as a function of the pseudo reduced pressure, can be used to determine the Z−factor.
For instance, for the gas composition listed in table 1.1, and at a pressure of 2000 psia and temperature of 180° F, the reader can verify that
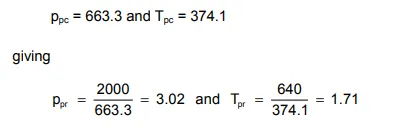
from which, using fig.1.6, the Z−factor can be obtained as 0.865.