DETERMINATION OF THE BASIC PVT PARAMETERS IN THE LABORATORY
These additional measurements will be briefly discussed in section 2.6. For the moment, the essential experiments required to determine the three basic parameters will be detailed, together with the way in which the results of a PVT analysis must be modified to match the field operating conditions. The analysis consists of three parts: − flash expansion of the fluid sample to determine the bubble point pressure; − differential expansion of the fluid sample to determine the basic parameters Bo, Rs and Bg; − flash expansion of fluid samples through various separator combinations to enable the modification of laboratory derived PVT data to match field separator conditions.
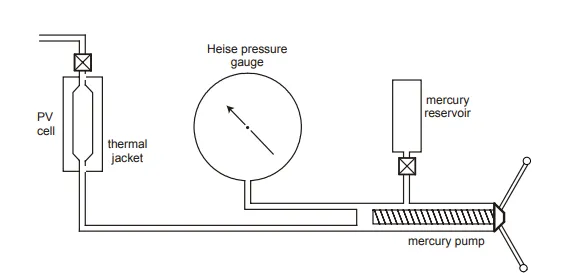
The apparatus used to perform the above experiments is the PV cell, as shown in fig. 2.8. After recombining the oil and gas in the correct proportions, the fluid is charged to the PV cell which is maintained at constant temperature, the measured reservoir temperature, throughout the experiments. The cell pressure is controlled by a positive displacement mercury pump and recorded on an accurate pressure gauge. The plunger movement is calibrated in terms of volume of mercury injected or withdrawn from the PV cell so that volume changes in the cell can be measured directly.
The flash and differential expansion experiments are presented schematically in figs. 2.9(a) and 2.9(b). In the flash experiment the pressure in the PV cell is initially raised to a value far in excess of the bubble point. The pressure is subsequently reduced in stages, and on each occasion the total volume vt of the cell contents is recorded. As soon as the bubble point pressure is reached, gas is liberated from the oil and the overall compressibility of the system increases significantly.
Thereafter, small changes in pressure will result in large changes in the total fluid volume contained in the PV cell. In this manner, the flash expansion experiment can be used to "feel" the bubble point. Since the cell used is usually opaque the separate volumes of oil and gas, below bubble point pressure, cannot be measured in the experiment and therefore, only total fluid volumes are recorded. In the laboratory analysis the basic unit of volume, against which all others are compared, is the volume of saturated oil at the bubble point, irrespective of its magnitude. In this chapter it will be assumed, for consistency, that this unit volume is one reservoir barrel of bubble point oil (1−rbb).

The bubble point pressure for this sample is determined from the flash expansion as 3330 psia, for which the saturated oil is assigned the unit volume. The relative total fluid volumes listed are volumes measured in relation to this bubble point volume. The flash expansion can be continued to much lower pressures although this is not usually done since the basic PVT parameters are normally obtained from the differential liberation experiment. Furthermore, the maximum volume to which the cell can expand is often a limiting factor in continuing the experiment to low pressures.