TBP/ASTM distillation curves
The most important characterization properties of the crude/intermediate/product streams are the TBP/ASTM distillation curves. Both these distillation curves are measured at 1 atm pressure. In both these cases, the boiling points of various volume fractions are being measured. However, the basic difference between TBP curve and ASTM distillation curve is that while TBP curve is measured using batch distillation apparatus consisting of no less than 100 trays and very high reflux ratio, the ASTM distillation is measured in a single stage apparatus without any reflux. Therefore, the ASTM does not indicate a good separation of various components and indicates the operation of the laboratory setup far away from the equilibrium.
Viscosity
Viscosity is a measure of the flow properties of the refinery stream. Typically in the refining industry, viscosity is measured in terms of centistokes (termed as cst) or saybolt seconds or redwood seconds. Usually, the viscosity measurements are carried out at 100 o F and 210 o F. Viscosity is a very important property for the heavy products obtained from the crude oil. The viscosity acts as an important characterization property in the blending units associated to heavy products such as bunker fuel. Typically, viscosity of these products is specified to be within a specified range and this is achieved by adjusting the viscosities of the streams entering the blending unit.
Flash and fire point
Flash and fire point are important properties that are relevant to the safety and transmission of refinery products. Flash point is the temperature above which the product flashes forming a mixture capable of inducing ignition with air. Fire point is the temperature well above the flash point where the product could catch fire. These two important properties are always taken care in the day to day operation of a refinery.
Pour point
When a petroleum product is cooled, first a cloudy appearance of the product occurs at a certain temperature. This temperature is termed as the cloud point. Upon further cooling, the product will ceases to flow at a temperature. This temperature is termed as the pour point. Both pour and cloud points are important properties of the product streams as far as heavier products are concerned. For heavier products, they are specified in a desired range and this is achieved by blending appropriate amounts of lighter intermediate products.
Octane number
Though irrelevant to the crude oil stream, the octane number is an important property for many intermediate streams that undergo blending later on to produce automotive gasoline, diesel etc. Typically gasoline tends to knock the engines. The knocking tendency of the gasoline is defined in terms of the maximum compression ratio of the engine at which the knock occurs. Therefore, high quality gasoline will tend to knock at higher compression ratios and vice versa. However, for comparative purpose, still one needs to have a pure component whose compression ratio is known for knocking. Iso-octane is eventually considered as the barometer for octane number comparison. While iso-octane was given an octane number of 100, nheptane is given a scale of 0. Therefore, the octane number of a fuel is equivalent to a mixture of a iso-octane and n-heptane that provides the same compression ratio in a fuel engine. Thus an octane number of 80 indicates that the fuel is equivalent to the performance characteristics in a fuel engine fed with 80 vol % of isooctane and 20 % of n-heptane. Octane numbers are very relevant in the reforming, isomerisation and alkylation processes of the refining industry. These processes enable the successful reactive transformations to yield long side chain paraffins and aromatics that possess higher octane numbers than the feed constituents which do not consist of higher quantities of constituents possessing straight chain paraffins and non-aromatics (naphthenes).
Crude chemistry
Fundamentally, crude oil consists of 84 – 87 wt % carbon, 11 – 14 % hydrogen, 0 – 3 wt % sulphur, 0 – 2 wt % oxygen, 0 – 0.6 wt % nitrogen and metals ranging from 0 – 100 ppm. Understanding thoroughly the fundamentals of crude chemistry is very important in various refining processes. The existence of compounds with various functional groups and their dominance or reduction in various refinery products is what is essentially targeted in various chemical and physical processes in the refinery. Based on chemical analysis and existence of various functional groups, refinery crude can be broadly categorized into about 9 categories summarized as
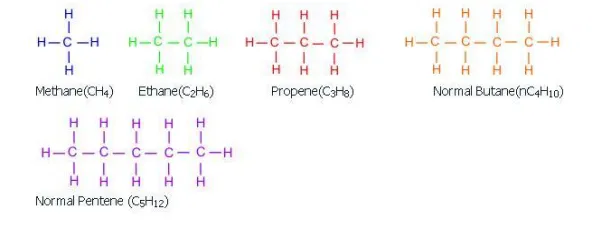
Paraffins:
Paraffins refer to alkanes such as methane, ethane, propane, n and iso butane, n and iso pentane. These compounds are primarily obtained as a gas fraction from the crude distillation unit.

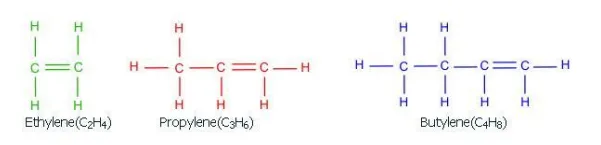
Olefins:
Alkenes such as ethylene, propylene and butylenes are highly chemically reactive. They are not found in mentionable quantities in crude oil but are encountered in some refinery processes such as alkylation.
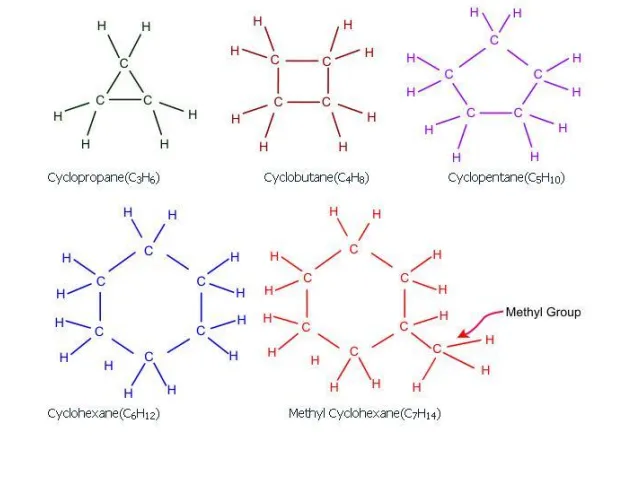
Naphthenes:
Naphthenes or cycloalkanes such as cyclopropane, methyl cyclohexane are also present in the crude oil. These compounds are not aromatic and hence do not contribute much to the octane number. Therefore, in the reforming reaction, these compounds are targeted to generate aromatics which have higher octane numbers than the naphthenes.
Napthalenes:
Polynuclear aromatics such as naphthalenes consist of two or three or more aromatic rings. Their molecular weight is usually between 150 – 500.

Organic sulphur compounds:
Not all compounds in the crude are hydrocarbons consisting of hydrogen and carbon only. Organic sulphur compounds such as thiophene, pyridine also exist in the crude oil. The basic difficulty of these organic sulphur compounds is the additional hydrogen requirements in the hydrotreaters to meet the euro III standards. Therefore, the operating conditions of the hydrotreaters is significantly intense when compared to those that do not target the reduction in the concentration of these organic sulphur compounds. Therefore, ever growing environmental legislations indicate technology and process development/improvement on the processing of organic sulphur compounds.
Oxygen containing compounds:
These compounds do not exist 2 % by weight in the crude oil. Typical examples are acetic and benzoic acids. These compounds cause corrosion and therefore needs to be effectively handled.
Resins: Resins are polynuclear aromatic structures supported with side chains of paraffins and small ring aromatics. Their molecular weights vary between 500 – 1500. These compounds also contain sulphur, nitrogen, oxygen, vanadium and nickel.
Asphaltenes: Asphaltenes are polynuclear aromatic structures consisting of 20 or more aromatic rings along with paraffinic and naphthenic chains. A crude with high quantities of resins and asphaltenes (heavy crude) is usually targeted for coke production.