Formation damage from paraffins and asphaltenes
Perhaps the most common formation damage problem reported in the mature oil-producing regions of the world is organic deposits forming both in and around the wellbore. These organic deposits fall into two broad categories:
· Paraffins
· Asphaltenes
These deposits can occur in tubing, or in the pores of the reservoir rock. Both effectively choke the flow of hydrocarbons. This article discusses the source, deposition, removal, and prevention of these deposits.
Paraffin and asphaltenes in crude oil
Crude oils contain three main groups of compounds:
· Saturated hydrocarbons or paraffins
· Aromatic hydrocarbons
· Resins and asphaltenes
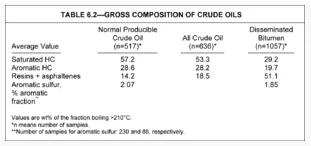
Table 1 shows the gross composition of crude oils, tars, and bitumens obtained from various sources. It is evident that crude oils contain substantial proportions of saturated and aromatic hydrocarbons with relatively small percentages of resins and asphaltenes. More degraded crudes, including tars and bitumens, contain substantially larger proportions of resins and asphaltenes.

Table 1
Paraffin deposition
Paraffins are high-molecular-weight alkanes (C20+) that can build up as deposits in the wellbore, in feed lines, etc. These organic deposits can act as chokes within the wellbore, resulting in a gradual decrease in production with time as the deposits increase in thickness. This can result in producing problems unless some remedial action is taken on a systematic and periodic basis. Deposits vary in consistency from soft accumulations to hard, brittle deposits. Usually the deposits are firmer and harder as the molecular weight of the paraffin deposits increases. Sometimes paraffins and asphaltenes occur together in organic deposits.
The primary cause of wax or paraffin deposition is simply a loss in solubility in the crude oil. [1][2] This loss of solubility is usually a result of changes in temperature, pressure, or composition of the crude oil as a result of loss of dissolved gases. paraffins that have the highest melting point and molecular weight are usually the first to separate from solution, with lower-molecular-weight paraffins separating as the temperature decreases further. For example, a C60 alkane with a melting point of about 215°F will deposit at a much higher temperature than a C20 alkane with a melting point of 98°F.
The ability of the crude oil to hold the paraffin in solution is generally quantified with two indicators:
· Pour point
· Cloud point
The procedure for measuring the pour point and cloud point may be found in ASTM manuals (D2500-66 for cloud points and D97-66 for pour points). The cloud point is defined as the temperature at which paraffins begin to come out of solution and a clear solution of hydrocarbons turns cloudy. Obviously, it is difficult to measure the cloud point for dark crude oil, because cloudiness is not visible. In such cases, the presence of paraffin crystals may have to be detected with a polarizing light microscope. The pour point is defined as the temperature at which the crude oil no longer flows from its container. As the temperature is lowered, wax crystals form an interlocking network that supports the hydrocarbon liquid within it. This network of paraffin crystals is quite shear sensitive and loose when first formed but can harden and become extremely rigid as fluid is lost from it. Pour points are relatively easy to measure in the field and provide a good indication of conditions under which large quantities of paraffin will fall out of solution in crude oils.
The most common cause of loss of solubility of the paraffin in the crude oil is a decrease in temperature, which may occur for a variety of reasons:
· Cooling produced by the crude oil and associated gas expanding through the perforations
· Gas expansion while lifting fluids to the surface
· Radiation of heat from the tubing to the surrounding formation induced by intrusion of water into or around the wellbore
· Loss of lighter constituents in the crude oil because of vaporization
Several other possible reasons for a decrease in temperature can be envisioned. In offshore installations, for example, paraffin problems are usually associated with the rapid change in temperature as the crude oil from the wellbore enters subsea pipelines that are immersed in seawater at 4°C. Large volumes of paraffins can be deposited on the surfaces of the pipelines, which requires periodic pigging. See Flow assurance for offshore and subsea facilities.
Pressure itself has little or no influence on the solubility of paraffin in crude oil. However, it does have a significant impact on the composition of the crude oil. Reductions in pressure usually lead to loss of volatiles from the crude oil and can induce the precipitation of paraffins. This is the primary reason why paraffin problems are more common in the more mature regions of the world. As the reservoir pressure is depleted and the lighter components of the crude oil are produced in preference to the heavier fractions, the likelihood of paraffin precipitation is significantly increased.
For paraffin deposition to be a significant problem, the paraffin must deposit on the pore walls or the tubing surface. If the paraffin remains entrained in the crude oil, it usually offers few production problems. Several factors influence the ability of paraffin to deposit on the pipe walls:
· Presence of water wetting the surfaces of the pipe tends to inhibit paraffin deposition. In addition, water has a higher specific heat than oil, which increases flowing temperatures.
· Pipe quality plays an important role. Rusty pipes with large surface area and numerous sites for paraffin crystal formation offer an ideal location for paraffin deposition. Paraffin adheres to rough surfaces better than smooth surfaces.
· Temperature profile in the near-wellbore region or within the pipe plays an important role in determining whether the paraffin will deposit on the walls or will continue to be entrained with the fluid.
The injection of fluids such as stimulation fluids or injection water into the wellbore can often induce paraffin deposition problems. This is particularly true if the surface temperature is significantly colder than the reservoir temperature. Field cases documenting paraffin precipitation during fracture stimulation are provided in McClaflin[1].
Removal of paraffin deposits
Paraffin accumulations are removed by methods that can be broadly placed into three categories:
· Mechanical removal of paraffin deposits
· Use of solvents to remove paraffin deposits
· Use of heat to melt and remove the wax
Mechanical methods such as scrapers, knives, and other tools are most commonly used to remove paraffin deposits in the wellbore. They can be very effective and are relatively inexpensive.
The most common solvent used to remove paraffin from tubulars and the near-wellbore region is crude oil. Hot oiling is the least expensive method, commonly used on stripper wells to remove paraffin deposits. Lease crude taken from stock-tank bottoms is heated to temperatures of 300°F or more. This heated oil is then injected or gravity fed into the tubing or annulus (more common). The high temperature induces solubilization of the paraffin deposits in the injected crude, which is then produced back to the surface. Hot oiling has been used successfully to remove paraffin deposition, but can result in formation damage. The use of hot salt water to melt the paraffin may be a safer approach.
Solvents, both organic and inorganic, have been used in the past. These include crude oil, kerosene, diesel, and surfactant formulations that can solubilize the paraffin. Organic solvents that consist of a blend of aromatics are usually used to remove mixtures of paraffin and asphaltene deposits. However, the cost of such treatments can be significantly higher than that of hot oil or water treatments.
Steam has been used in a number of cases in which severe paraffin problems have resulted in plugged tubulars. The lack of solubility of paraffin in hot water necessitates the use of surfactants with steam or hot water so that the melted paraffin can be removed.
Methods for preventing paraffin deposition
Several mechanical adjustments can be made in the production string that can minimize the likelihood of paraffin deposition. In general, these steps are designed to minimize the cooling of the crude oil as it is produced to the surface. This can be accomplished by designing pumping wells or tubing sizes and gas lift systems that maximize the flow of oil to the surface and minimize the heat lost to the surrounding formations. Use of more expensive methods such as plastic coatings on tubulars and electrical heaters is severely limited by economics.
Paraffin inhibitors are a class of compounds that consist of crystal modifiers that prevent the deposition of paraffin onto pipe surfaces. These surface-active materials retard paraffin deposition by inhibiting the adhesion of paraffin to sites on the tubing walls. Surfactants used in these applications include wetting agents, dispersants, and crystal modifiers. [3][4] Each of these chemicals needs to be tested for a specific crude oil to evaluate its effectiveness.
Asphaltene precipitation
High-molecular-weight constituents of crude oil containing nitrogen, sulfur, and oxygen (N, S, and O) compounds are referred to as asphaltenes. This broad class of compounds is clearly not hydrocarbon because these compounds contain a large portion of heteroatoms in their structure. Lower-molecular-weight NSO compounds are referred to as resins. The separation of crude oil into resins and asphaltenes and other constituents is based primarily on solubility. Asphaltenes and resins are generally defined as the pentane-insoluble fraction of the crude oil. [5]
The average molecular structure of an example asphaltene fraction from a crude oil from Venezuela is shown in Fig. 1.
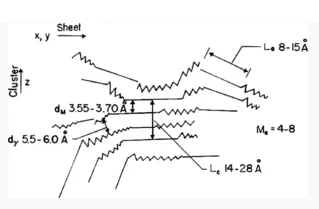
Fig. 1—Cross-sectional view of an asphaltene model based on X-ray diffraction. Zigzag line represents configuration of a saturated carbon chain or loose net of naphthenic rings; straight line represents the edge of flat sheets of condensed aromatic rings.[5]
It consists primarily of condensed aromatic rings associated with aliphatic tails. The polynuclear aromatic rings associate with each other through their π electron systems to form clusters of stacked rings, as shown in the figure. In crude oils, these asphaltene structures are dispersed and maintained in suspension by the action of resins. If sufficient quantities of resin molecules are present in the crude oil, the asphaltenes remain dispersed and in solution. However, the addition of large quantities of alkanes or removal of the resin fraction can result in a loss of solubility because the asphaltene molecules associate with each other, forming large aggregates or micelles, and precipitate out. These micelles or aggregates are visible under optical microscopes as dark, solid aggregates. Precipitation of asphaltenes occurs through the formation of such aggregates. The solubility of asphaltenes is therefore a function of temperature, pressure, and the composition of the crude oil. Any action that affects the compositional balance of the crude oil can affect the ability of the oil to maintain the asphaltenes in solution.
A very common example of the change in composition of a crude oil is what occurs during pressure depletion in a reservoir. As shown in Fig. 2, the solubility of asphaltene is a minimum at the bubblepoint pressure. [6] This has important consequences for predicting where asphaltene precipitation will occur in a reservoir. As the reservoir is depleted and the bubblepoint pressure is achieved lower in the tubing or even in the formation itself, the possibility of asphaltene deposition occurs at these locations. Indeed, in studies published in the literature, the location of asphaltene deposition is observed to move from the top of the tubing to the bottom and into the reservoir over a period of time as the reservoir pressure is depleted and the location where the bubblepoint pressure is reached moves further out toward the reservoir.
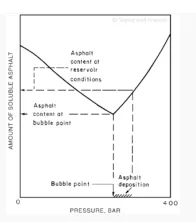
Fig. 2—Pressure dependence of asphalt solubility for a North Sea crude oil showing the possibility of asphalt deposition in the well tubing.[6]
Asphaltene deposition can also be induced by changes in composition of the crude oil through injection of fluids such as CO2 or lean gas. [7][8] Several studies have documented the possibility of asphaltene precipitation during lean gas and CO2 injection [9][10]. Large changes in temperature can also induce asphaltene deposition. [11][12] In such cases, deposits of paraffin and asphaltene are commonly observed together. The asphaltene particles frequently act as nucleation sites for paraffin crystals.