Scale problems in production
Wells producing water are likely to develop deposits of inorganic scales. Scales can and do coat perforations, casing, production tubulars, valves, pumps, and downhole completion equipment, such as safety equipment and gas lift mandrels. If allowed to proceed, this scaling will limit production, eventually requiring abandonment of the well.
Technology is available for removing scale from tubing, flowline, valving, and surface equipment, restoring at least some of the lost production level. Technology also exists for preventing the occurrence or reoccurrence of the scale, at least on a temporary basis. “Temporary” is generally 3 to 12 months per treatment with conventional inhibitor “squeeze” technology, increasing to 24 or 48 months with combined fracture/inhibition methods. This page discusses types of inorganic scale, their control, inhibition, and removal.
Scale mechanisms
As brine, oil, and/or gas proceed from the formation to the surface, pressure and temperature change and certain dissolved salts can precipitate. This is called “self-scaling.” If a brine is injected into the formation to maintain pressure and sweep the oil to the producing wells, there will eventually be a commingling with the formation water. Additional salts may precipitate in the formation or in the wellbore (scale from “incompatible waters”). Many of these scaling processes can and do occur simultaneously. Scales tend to be mixtures. For example, strontium sulfate is frequently found precipitated together with barium sulfate. The chemical formulae and mineral names for most oilfield scales are shown in Table 1.

Table 1 - Oilfield mineral scales
The most common oilfield scales are:
· Calcite
· Barite
· Celestite
· Anhydrite
· Gypsum
· Iron sulfide
· Halite
“Exotic” scales such as calcium fluorite, zinc sulfide, and lead sulfide are sometimes found with high temperature/high pressure (HT/HP) wells.
Calcite deposition is generally a self-scaling process. The main driver for its formation is the loss of CO2 from the water to the hydrocarbon phase(s) as pressure falls. This removes carbonic acid from the water phase, which had kept the basic calcite dissolved. Calcite solubility also decreases with decreasing temperature (at constant CO2 partial pressure).
Halite scaling is also a self-scaling process. The drivers are falling temperature and evaporation. Halite solubility in water decreases with decreasing temperature, favoring halite dropout during the production of high total dissolved solids (TDS) brines to the surface. (Falling pressure has a much smaller effect on decreasing halite solubility.) Evaporative loss of liquid water is generally the result of gas breakout from undersaturated condensate and oil wells, as well as the expansion of gas in gas wells. This increase in water vapor can leave behind insufficient liquid water to maintain halite solubility in the coproduced brine phase. Halite self-scaling is found with both high-temperature and low-temperature wells [e.g., with 125 and 350°F bottomhole temperature (BHT) gas/gas condensate wells].
Barite scales are generally the result of mixing incompatible waters. For example, seawater is often injected into offshore reservoirs for pressure maintenance. Seawater has a high-sulfate content; formation waters often have high-barium contents. Mixing these waters results in barite deposition. If this mixing/precipitation occurs within the reservoir far removed from a vertical wellbore, there will generally be little impact on the production of hydrocarbons. Mixing/precipitation near or within the wellbore will have a significant impact on production. Mixing of incompatible waters within the sandpack of a hydraulically fractured well can also be detrimental to production. Furthermore, after the initial, large deposition of scale, this water continues to be saturated in barite and additional barite scale will continue to form in the wellbore as pressure and temperature fall.
Waterfloods combining ground waters with high calcium and high sulfate contents can deposit anhydrite or gypsum by much the same “incompatible waters” mechanism discussed for barite. However, calcium sulfate scale solubility, unlike that of barite scale, actually increases with decreasing temperature (until about 40°C). This can decrease the likelihood of scale after the initial mixing deposition. The reversal in solubility falloff below 40°C accounts for the gypsum scaling observed in surface equipment. This inverse temperature effect can result in the generation of anhydrite scale when injecting seawater. Anhydrite solubility falls as pressure falls; data could not be found for gypsum solubility vs. pressure.
Iron sulfide scales are almost ubiquitous when hydrogen sulfide is produced—frequently the result of tubular corrosion in the presence of H2S. A review of the iron sulfide chemistry and phases occurring in production equipment is contained in Nasr-El-Din and Al-Humaidan[2] and Cowan and Weintritt.[3] The chemistry is complicated; more than one iron sulfide phase can be present. The physical properties of the phases vary (sometimes dense, sometimes not), and the phase composition can change with time.
These multistep scale/water chemistries can be simulated with present day computer software. Some of the programs are commercial, and some operators have their own in-house programs. In effect, the code sets up a series of equilibrium equations for each possible scale and solution ion/ion reaction, as well as solution-gas reaction, then solves them simultaneously as a function of:
· Input pressure
· Temperature
· Gas composition
· Water-phase composition
These are referred to as “thermodynamic models.” Software has not yet reached a level of sophistication sufficient to say, reliably, how fast these solids can form during production. This has resulted in a series of “rules-of-thumb,” correlating an operator’s field experience with the thermodynamic simulator’s output. Such rules of thumb are much less necessary for formation scaling, particularly if the mineral is naturally present in the formation (e.g., calcite). Computer simulation of scaling tendencies for produced oilfield brines has found considerable acceptance and application. Examples of this technology, applied to halite and calcite scaling in HT/HP wells, are in Jasinski, et al. and Jasinski.
Economic considerations
Scale remediation and prevention comes at a cost. It is more appropriate to think of scale control not as a cost, but in terms of “value added”—obviating the consequences of not remediating or preventing scale formation, and so increasing the total revenue from a well, as well as possibly extending its lifetime. The effects of scale can be quite expensive and rapid. In one North Sea well (Miller field), production fell from 30,000 B/D to zero in just 24 hours because of scaling. The cost for cleaning out the single well and putting it back on production was approximately the same as the chemical costs to treat the entire field. While not all wells are susceptible to such momentous penalties for allowing scaling to initiate, there is no question that scale formation, remediation, and prevention have associated costs. The cost savings because of less deferred/lost oil can result in substantially increased revenue over the life of the well, as well as more oil.
It is anticipated that oilfield scaling problems will continue to worsen and become more expensive.[8] The new drivers are:
· Tendency to longer tiebacks
· Use of smart wells (integrity more critical)
· More gas production (gas well formations tend to be more delicate)
· Nneed to use greener chemicals
· Increasing amounts of produced water
Identifying scale occurrence
Scale control has tended to be reactive rather than proactive. There are a variety of methods of removing the effects of scale on production. The first step is to determine which scales are forming and where they are forming. Some of this information can be reliably inferred from the computer simulation procedures discussed above, particularly for self-scaling processes. The simplest method of physically detecting scale in the wellbore is to run calipers down the wellbore and measure decreases in the tubing inner diameter. Gamma ray log interpretation has been used to indicate barium sulfate scale because naturally radioactive radium (Ra226) precipitates as an insoluble sulfate with this scale. An example of this technology is shown in Fig. 1. Visual observation with the appropriate wireline tools has also been used to show the presence of calcite and halite solids within the wellbore.
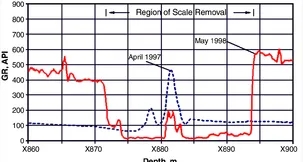
Fig. 1—The 1997 gamma ray log shows the buildup on the lower side-pocket mandrel one year before treatment. The 1998 log was measured after the scale was removed from the zone between X872 and X894m (after Schlumberger Oilfield Review).
The onset of water production coinciding with simultaneous reduction in oil production is a sign of potential scale problems. It is quite possible, particularly with gas wells, to produce water below the limit of detection of surface analysis (nominally 1 or 2%). This water will evaporate and leave its dissolved solids behind, as scale. Because the amounts of water are small, the amounts of solids per unit volume of water will be small, but the solids will accumulate with time. The same idea applies to the appearance at the surface of liquid “fresh” water when the reservoir brine is known to be brackish. This can be condensed water because of falling temperature. When a few percent of liquid water is produced, it is prudent to track the dissolved ion content with time. Injection-water breakthrough is generally signaled by dramatic changes in the concentrations of scaling ions, such as barium or sulfate, which coincide with reduced oil production.
Early warning of scaling conditions downhole would be valuable. Wells with intelligent completions and permanent monitoring systems are being designed to contain scale sensors. The function of the scale sensor is double duty—not only to provide early warning about the initiation of production impairment by scale generation but also to provide information about possible impairment of the smart-well sensors and valves by films of scale.