Classification of Reservoirs
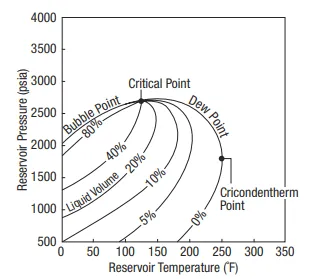
All hydrocarbon mixtures can be described by a phase diagram such as the one shown in Figure 1-3. Plotted are temperature (x axis) and pressure (y axis). A specific point is the critical point, where the properties of liquid and gas converge. For each temperature less than the critical-point temperature (to the left of in Figure 1-3) there exists a pressure called the “bubble-point” pressure, above which only liquid (oil) is present and below which gas and liquid coexist.
For lower pressures (at constant temperature), more gas is liberated. Reservoirs above the bubble-point pressure are called “undersaturated.” If the initial reservoir pressure is less than or equal to the bubble-point pressure, or if the flowing bottomhole pressure is allowed to be at such a value (even if the initial reservoir pressure is above the bubble point), then free gas will at least form and will likely flow in the reservoir. This type of a reservoir is known as “two-phase” or “saturated.”

For temperatures larger than the critical point (to the right of in Figure 1-3), the curve enclosing the two-phase envelop is known as the “dew-point” curve. Outside, the fluid is gas, and reservoirs with these conditions are “lean” gas reservoirs.
The maximum temperature of a two-phase envelop is known as the “cricondentherm.” Between these two points there exists a region where, because of the shape of the gas saturation curves, as the pressure decreases, liquid or “condensate” is formed. This happens until a limited value of the pressure, after which further pressure reduction results in revaporization.
The region in which this phenomenon takes place is known as the “retrograde condensation” region, and reservoirs with this type of behavior are known as “retrograde condensate reservoirs.” Each hydrocarbon reservoir has a characteristic phase diagram and resulting physical and thermodynamic properties.
These are usually measured in the laboratory with tests performed on fluid samples obtained from the well in a highly specialized manner. Petroleum thermodynamic properties are known collectively as PVT (pressure–volume–temperature) properties.