Surface and lnterfaclal Tensions
The term interface indicates a boundary or dividing line between two immiscible phases. Types of interfaces include: liquid-gas, liquid-liquid, liquidsolid, solid-gas, and solid-solid. For fluids, molecular interactions at the interface result in a measurable tension which, if constant, is equal to the surface free energy required to form a unit area of interface. For the case of a liquid which is in contact with air or the vapor of that liquid, the force per unit length required to create a unit surface area is usually referred to as the surface tension. Interfacial tension is used to describe this quantity for two liquids or for a liquid and a solid.
Interfacial tension between two immiscible liquids is normally less than the surface tension of the liquid with the higher tension, and often is intermediate between the individual surface tensions of the two liquids of interest. Common units of surface or interfacial tension are dynes per centimeter (or the identical ergs/cm*) with metric units in the equivalent milli-Newton per meter (mN/m). The surface tension of pure water ranges from 72.5 dynes/cm at 70°F to 60.1 dynes/cm at 200°F in an almost linear fashion with a gradient of 0.095 dynes/cm/"F [25]. Salts in oilfield brines tend to increase surface tension, but surface active agents that may dissolve into the water from the oil can lower surface tension.
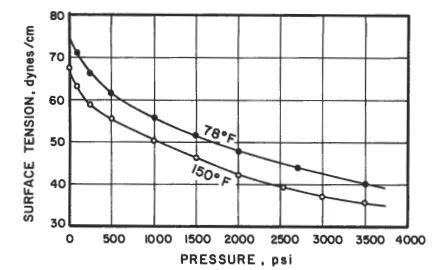
At standard conditions, surface tensions of brines range from 59 to 76 dynes/cm [25]. As shown in Figure 5-39, dissolved natural gas reduces surface tension of water as a function of saturation pressure [89]. At a given temperature, surface tension of hydrocarbons in equilibrium with the atmosphere or their own vapor increases with increasing molecular weight (Figure 5-40) [go]. For a given hydrocarbon, surface tension decreases with increasing temperature. At 70°F, surface tensions of crude oils often range from 24 to 38 dyne/cm [25].
The presence of dissolved gases greatly reduces surface tension of crude oil as shown in Figure 5-41 [91]. Dissolved natural gas reduces surface tension of crude oil more than previously noted for water, but the amount and nature of gas determines the magnitude of the reduction. The direct effect of a temperature increase on reduction of surface tension more than counterbalances the decreased gas solubility at elevated temperatures.
Thus, surface tension at reservoir temperature and pressure may be lower than indicated by figure 541 [25]. Under reservoir conditions, the interfacial interaction between gas and oil involves the surface tension of the oil in equilibrium with the gas. Similarly, the interaction between oil and water determines the interfacial tension between the crude and brine. Listed in Table 5-14 are the surface and interfacial tensions for fluids from several Texas fields [92]. The effect of temperature on interfacial tensions for some oil-water systems is shown in Figure 5-42 [92]; the reduction in interfacial tension with increasing.

temperature is usually somewhat more pronounced than is observed for surface tension. Although no quantitative relation is observed, the general trend suggests lower interfacial tensions for the higher API gravity crudes. However, in studies with a crude oil containing large amounts of resins and asphaltenes, different effects of temperature on interfacial tension were observed when measurements made at aerobic conditions were compared to anaerobic tests [93]. Interfacial tension between the crude and reservoir brine showed a decrease with an increase in temperature under aerobic conditions, whereas at anaerobic conditions, interfacial tension increased with increasing temperatures.
This difference in behavior was attributed to oxidation of the stock tank oil in the aerobic tests. At conditions of reservoir temperature and pressure, interfacial tension of the live reservoir oil was higher than the stock tank oil. The study concluded that live reservoir crude should be used in measurements of interfacial properties and that if stock tank oil is used, at least the temperature should correspond to reservoir conditions. Figure 543 shows the effect of dissolved gas and pressure on the interfacial tension of three oil-water systems [89]. For each system, interfacial tension increases as the amount of dissolved gas increases, but drops slightly as pressure is increased above the bubblepoint.
Surface and interfacial tensions are important in governing the flow of fluids in the small capillaries present in oil-bearing reservoirs. The capillary forces in oil or gas reservoirs are the result of the combined effect of surface and interfacial tensions, pore size distribution, pore shape, and the wetting properties of the hydrocarboqhxk system.