Geochemistry of Carbonate Minerals
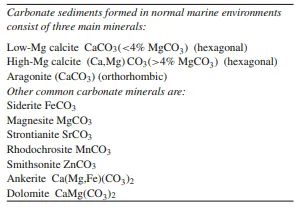
Carbonate minerals consist of CO2− 3 and one or more cations. The most common cations in carbonate minerals together with their mineral names are listed in Table 5.1. The common rock-forming carbonate minerals are either rhombohedral (calcite) or orthorhombic (aragonite) in crystal habit. Where cations with small ionic radii are incorporated into carbonates a trigonal

(rhombohedral) crystal lattice is formed, while larger cations result in orthorhombic unit cells. Ca2+ has an ionic radius close to 1 Å which is intermediate between small and large cations and near the limit of sixfold co-ordination.
Thus CaCO3 is dimorphous forming either rhombohedral or orthorhombic structures. Cations smaller than 1 Å such as Fe2+, Mn2+, Zn2+ and Mg2+ can be incorporated in the calcite lattice. These metals all have an ionic radius of about 0.6–0.7 Å, and therefore calcite can contain considerable concentrations of these cations. The calcite group of minerals, such as siderite (FeCO3), rhodochrosite (MnCO3), smithsonite (ZnCO3) and magnesite (MgCO3), all have the same crystal structure as calcite. Two types of calcite are recognised, depending on the magnesium content: low-Mg calcite (<4 mol% MgCO3) and high-Mg calcite (>4 mol% MgCO3). Biologically secreted calcite is high-Mg calcite and typically ranges between 11 and 19 mol% MgCO3. Low-Mg calcite (in most cases simply called calcite) is more stable than high-Mg calcite, and fossil fragments originally composed of high-Mg calcite are converted to low-Mg calcite during diagenesis.
The alteration of high-Mg calcite to low-Mg calcite takes place by a process of leaching of Mg2+ ions, which leaves the microarchitecture of the grain unaffected. The exsolved Mg2+ may form microdolomite rhombs that are sometimes seen as inclusions in calcitised high-Mg calcites (quite common in fragments of echinoderms and calcareous red algae). The orthorhombic lattice has an arrangement of CO2− 3 anions where cations larger than 1 Å (such as Sr2+, Ba2+ and Pb2+) are preferred.
Analogous with this aragonite crystal structure are strontianite (SrCO3), witherite (BaCO3) and cerrusite (PbCO3). Sr in particular is an important trace element in aragonite. Aragonite crystals forming in marine environments today contain 5,000–10,000 ppm Sr. Aragonite is unstable and after some time will be replaced by calcite which still retains relatively high concentrations of strontium. Aragonite may occasionally be preserved, particularly in dense shales, even in Mesozoic rocks. Iron is only very weakly soluble in the oxidised state, forming hydroxides Fe(OH)3 and oxides (Fe2O3), but in the reduced state it occurs as soluble Fe2+. Reduction of iron normally takes place within the microbial sulphate reduction zone where high concentrations of sulphur will cause available Fe2+ to be precipitated as sulphides (pyrite, FeS2), so that very little is available to enter the calcite structure.
The principal environment in which Fe2+ can enter the calcite lattice to form ferroan calcite is thus in the reducing porewater below the sulphate reduction zone. The ferroan calcite may contain a few thousand ppm of iron. Dolomite (CaMg(CO3)2) is a carbonate mineral in which layers of CaCO3 alternate with layers of MgCO3. Fe2 + is commonly found substituting for Mg2+ in dolomite, and a complete series extends to ankerite (Ca(Fe,Mg)(CO3)2). Dolomite formed early in diagenesis is fine-grained and can often have a magnesium deficit in relation to calcium [e.g. Ca55Mg45(CO3)100]. This is called protodolomite which during burial may be transformed into a regular dolomite.