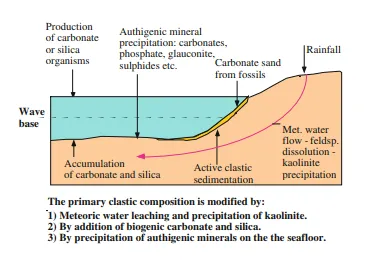
Meteoric Water Flow and Mineral Dissolution
Meteoric water is rainwater which infiltrates the ground. Initially this water is distilled water and therefore undersaturated with respect to all minerals. The reactions between meteoric water and the land surface are an important part of the weathering process. Rainwater contains carbon dioxide (CO2) and sulphur dioxide (SO2) from the air and is therefore slightly acidic, producing carbonic acid (H2CO3) and sulphuric acid (H2SO4). Some of the rainwater seeps down to the groundwater, and as long as the groundwater table is above sea level, meteoric water will flow along the most permeable beds into the basin.
Meteoric water will first dissolve carbonates and then slowly dissolve unstable minerals like feldspar and mica (Fig. 4.3). Decaying organic matter in the ground produces CO2 which is added to the groundwater, making it more acid. Humic acids generated by decaying plants also hasten the weathering reactions. At the same time this acidity is neutralised by weathering reactions with silicate minerals like feldspar and the dissolution of carbonates which consume protons (H+). As the groundwater reacts with minerals and in some cases with amorphous phases, it will approach equilibrium with many of the minerals present and this will happen first with carbonates. In the case of silicate minerals these reactions are very slow so the porewater may remain under- or supersaturated for a long time with respect to silicate minerals like quartz and feldspar.

ground and are a kind of subsurface weathering along the groundwater flow paths. Leaching by meteoric water is generally strong in fluvial and alluvial sediments. Even within dry river beds there is a focused flow of groundwater. The groundwater level represents the head (potentiometric surface) for groundwater flow and groundwater therefore has a potential to flow through sediments or other aquifers far below sea level.
The rates of leaching of minerals like feldspar and mica and the precipitation of kaolinite are functions of the flux of groundwater flowing through each rock volume per unit of time. These are in principle weathering reactions similar to those which take place during normal weathering in a humid climate. Cations like Na+ and K+ are stripped from silicate minerals like feldspar and mica and brought into solution. These reactions can be written as below:
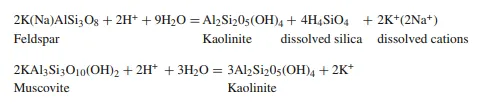
The porewater does not have to be acidic for kaolinite to form, but the K+/H+ ratio must be low. If the pH is high the K+ concentration has to be correspondingly lower. Authigenic kaolinite may also form in impure limestones as a result of meteoric water flushing, and the porewater is then certainly not acidic. Even if there is only a small amount of carbonate it will buffer the composition of the porewater.