Clay Minerals
A number of minerals are referred to as clay minerals because they predominantly occur in the finest grain-size fraction (clay fraction) of sediments and sedimentary rocks. However, this is not an accurate definition, because the clay fraction contains many other minerals than those we call clay minerals, and because the clay minerals themselves are often larger than 4 μm (0.004 mm). By “clay minerals” we usually mean sheet silicates which consist chiefly of oxygen, silicon, aluminium, magnesium, iron and water (H2O, OH–).
Clay minerals in sedimentary basins are partly derived from sheet silicate minerals occurring in metamorphic and eruptive rocks (e.g. biotite, muscovite and chlorite), but during weathering and transport these clastic minerals are typically altered from their initial composition in the parent rock. Mica (muscovite and biotite) lose some potassium which is replaced by water (H2O, H3O+) to form illite (hydro mica).
Clay minerals are also formed through weathering reactions, for example by the breakdown of feldspar and mica. Clay minerals which are formed by the breakdown of other minerals within the sediment, are called authigenic. Sheet silicates have a structure consisting of sheets of alternating layers of SiO4 tetrahedra and octahedra. In the tetrahedral layers, silicon or aluminium atoms are surrounded by four oxygen atoms. In the octahedral layers the cation is surrounded by six oxygen or hydroxyl ions.
Both bi and trivalent ions can act as cations in the octahedral layer. In sheet silicates with trivalent ions (e.g. Al3+) only two of the three positions in the octahedral layer are occupied, and such minerals are therefore called dioctahedral. With bivalent ions (Mg++, Fe++) all three positions must be filled to achieve a balance between the positive and negative charges, so these minerals are called trioctohedral.
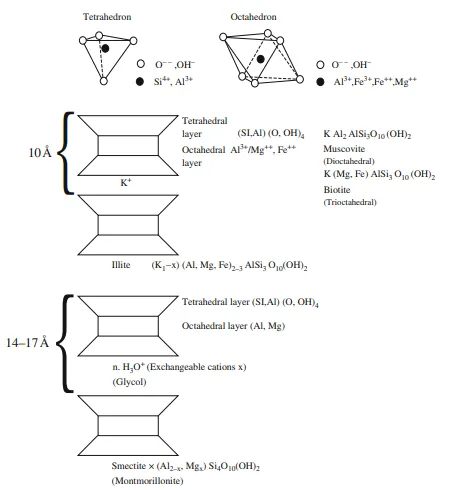
The main method of identifying clay minerals is X-ray diffraction (XRD), by which the thickness of the sheet silicates is determined using X-rays which are diffracted according to Bragg’s Law: nλ = 2d sin ϕ. Here λ is the wavelength of the X-ray, ϕ the angle of incidence and d the thickness of the reflecting silicate layers; d is thus a function of angle ϕ. Sheet silicates may also be identified by means of differential thermal analysis (DTA), which records characteristic exothermal or endothermal reactions. Figure 3.8 shows the structure of some of the main clay minerals. Illite consists of sheets with two layers of tetrahedra and one of octohedra, bonded together by potassium. This ionic bonding is relatively weak so the mineral cleaves easily along this plane.
The bonds within the tetrahedral and octahedral layers are more covalent and stronger. The potassium content in mica corresponding to the formula of mica is about 9% K2O, while illite has a greater or lesser deficit of potassium. Smectite (montmorillonite) has the same structure except that most of the potassium is replaced by water (H3O+), other cations or organic compounds (e.g. glycol). There are strong indications that smectite consists of small particles of 10 Å, plus water.
Illite is most likely comprised of several layers of these small 10 Å particles stacked on top of one another. In an atmosphere of glycol vapour, smectite will swell from 14 to 17 Å, while illite is unable to expand because there are numerous layers bonded together with K+ or other cations, for example NH+. Smectite has a very high ion-exchange capacity and to some extent can exchange ions in the octahedral layer. The stability of smectite declines in aqueous solutions with high K+/H+ Na+/H+ ratio and with increasing temperature, and it converts to illite. Vermiculite has a structure reminiscent of the smectites, and also undergoes ion exchange and thus charge deficit in the tetrahedral layer, so that the bonding between each layer is too strong for much swelling to occur.
Vermiculites are mostly trioctahedral, containing mostly Mg or Fe in the octahedral layer. Glauconite is a green mineral which forms on the seabed. It is a potassium and iron bearing silicate somewhat similar to illite and contains both di - and trivalent iron. It is therefore formed right on the redox boundary, and during periods with little or no clastic sedimentation this can result in relatively pure beds of glauconite.

Clay minerals have a number of properties which distinguish them from most other minerals. Because of their very large specific surface area they have a great capacity for adsorbing ions, which is increased by the fact that clay minerals have negatively charged edges due to broken bonds. In water with a low electrolyte content, clay minerals will therefore repel each other. If cations are added, clay minerals will then accumulate a layer of positive ions (a double layer), and repulsion between negatively charged clay minerals declines as the strength of the electrolyte increases. Van der Waal’s forces will therefore cause flocculation more easily in saltwater, where repulsion due to the negative charge is reduced. This is why clays transported by rivers flocculate into larger particles which sink more rapidly to the seafloor.