Sedimentary Geochemistry
The composition and physical properties of sedimentary rocks are to a large extent controlled by chemical processes during weathering, transport and also during burial (diagenesis). We can not avoid studying chemical processes if we want to understand the physical properties of sedimentary rocks. Sediment transport and distribution of sedimentary facies is strongly influenced by the sediment composition such as the content of sand/clay ratio and the clay mineralogy.
The primary composition is the starting point for the diagenetic processes during burial. We will now consider some simple chemical and mineralogical concepts that are relevant to sedimentological processes. Clastic sediments are derived from source rocks that have been disintegrated by erosion and weathering. The source rock may be igneous, metamorphic or sedimentary. The compositions of clastic sediments are therefore the product of the rock types within the drainage basin (provenance), of climate and relief.
The dissolved portion flows out into the sea or lakes, where it is precipitated as biological or chemical sediments. Weathering and abrasion of the grains continues during transport and sediments may be deposited and eroded several times before they are finally stored in a sedimentary basin. After deposition sediments are also being subjected to mineral dissolution and precipitation of new minerals as a part of the diagenetic processes.
For the most part we are concerned with reactions between minerals and water at relatively low temperatures. At temperatures above 200–250◦C these processes are referred to as metamorphism which is principally similar in that unstable minerals dissolve and minerals which are thermodynamically more stable at certain temperatures and pressures precipitate. At low temperatures, however, unstable minerals and also amorphous phases may be preserved for a long time and there may be many metastable phases.
Many of the reactions associated with the dissolution and precipitation of minerals proceed so slowly that only after an extremely long period can they achieve a degree of equilibrium. Reactions will always be controlled by thermodynamics and will be driven towards more stable phases. The kinetic reaction rate is controlled by temperature. Silicate reactions are very slow at low temperature and this makes it very difficult to study them in the laboratory. Biological processes often accompany the purely chemical processes, adding to the complexity. Bacteria have been found to play an important role in both the weathering and precipitation of minerals.
Their chief contribution is to increase reaction rates, particularly during weathering. In this chapter we shall examine the processes between water and sediments from a simple physicalchemical viewpoint. A detailed treatment of sediment geochemistry is however beyond the scope of this book.

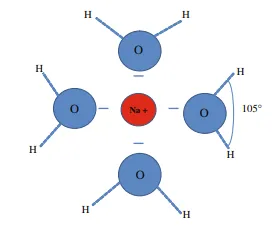
Water (H2O) consists of one oxygen atom linked to two hydrogen atoms, with the H-O-H bonds forming an angle of 105◦ (Fig. 3.1). The distance between the O and the H atoms is 0.96 Å, and between the hydrogen atoms 1.51 Å. Water molecules therefore have a strong dipole with a negative charge on the opposite side from the hydrogen atoms (Fig. 3.1). This is why water has a relatively high boiling point and high viscosity, and why it is a good solvent for polar substances. Another consequence of this molecular structure is that water has a high surface tension, important for enabling particles and organisms to be transported on its surface. The capillary forces which cause water to be drawn up through fine-grained soils are also a result of this high surface tension.
Ions with low ionic potential are unable to break the bonds in the water molecule and therefore remain in solution as hydrated cations (e.g. Na+, K+). This means that the ion is surrounded by water molecules with their negative dipole towards the cation (Fig. 3.1). This is because the O–H bond is stronger than the bond which the cation forms with oxygen (M–O bonding, M = metal); this is particularly true of alkali metal ions (Group I) and most alkaline earth elements (Group II, I.P. <3). Metals with an ionic potential only slightly lower than that required to form M–O bonds, namely Mg2+, Fe2+, Mn2+, Li+ and Na+, will be the most strongly hydrated. The hydration strongly affects the chemical properties of the ion and its capacity to be adsorbed or enter into the crystal structure of a mineral. Since the ions are surrounded by water molecules, we can use the expression “hydrated radius” to describe the space occupied by the ion and its water molecules within a crystal structure (Fig. 3.3). If the M–O bond is approximately equal in strength to the O–H bond (I.P. 3–12), the metal ion replaces one of the hydrogen atoms to form very low solubility compounds of the type M(OH)n (see Fig. 3.2). Examples of these so-called hydroxides that we commonly encounter in sedimentary rocks as a result of weathering are Fe(OH)3, Al(OH)3 and Mn(OH)4. These hydroxides have very low solubility. Ions with high ionic potential (>12) form an M–O bond that is stronger than the H–O bond, giving soluble anion complexes such as SO4 −−, CO3 −−, PO4 3− and releasing both of the H+ ions into solution.