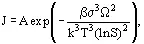
Processes Affecting Crystals
The driving force for both the formation of new crystals and the growth of existing ones is supersaturation. This arises from the concentration of solute exceeding the equilibrium (saturation) solubility concentration.
Thermodynamically, the driving force is the change in Gibbs’ free energy, Δμ = μ - μ* = RTlnγ/γ* , where γ is the activity coefficient. However, this driving force is difficult to determine so the concentration difference, Δc = c - c*, is most commonly used in practical correlations. Uncrystallized solute molecules start by being dispersed randomly in a solution or melt. Nucleation is the process whereby new crystals are formed. Primary nucleation occurs in crystal-free solution; its exact mechanism is not understood but probably involves the formation of semistructured clusters which rearrange to form crystal nuclei. Ideal primary homogeneous nucleation can be derived thermodynamically by considering the formation of liquid droplets from a vapor phase:
(1)
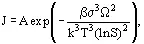
which indicates that the nucleation rate J is governed by the interfacial tension σ, the supersaturation ratio S = c/c*, and the temperature T, with the constant A, shape factor β, molecular volume Ω and Boltzmann's constant k all being constant for a given system. This equation can, in theory, be applied to the formation of idealized solid particles in a liquid phase. The equation has two practical problems: the interfacial tension is very difficult to measure (it is usually easiest to get it from the nucleation rate) and primary homogeneous nucleation is rare in industrial systems unless solutions and vessels are very clean. In practice, primary heterogeneous nucleation occurs in crystal-free solution, where crystals form on particles in suspension (e.g., dust) or secondary nucleation, where new crystals are formed from existing crystals in solution. As a result, pragmatic nucleation correlations of the form B = knΔcb (primary) or B = kbΔcb![]() (secondary) are often found. Measurement of nucleation rates is difficult, and usable values are rarely found in the literature; nucleation is the most difficult parameter to characterize for most systems.
(secondary) are often found. Measurement of nucleation rates is difficult, and usable values are rarely found in the literature; nucleation is the most difficult parameter to characterize for most systems.
Crystal growth occurs when solute molecules in solution diffuse to the surface of the crystal, become adsorbed onto the surface and are then incorporated into the crystal lattice. For some systems, incorporation of solute into the crystal is easy and growth is limited by diffusion to the crystal surface through bulk solution or boundary layer. For other systems, surface integration of solute is rate controlling and growth on a flat crystal face is difficult; growth mainly occurs on stepped or kinked edges. For extreme cases, growth is strongly dependent on dislocations in the crystal.
At low supersaturation levels, crystal growth occurs but primary nucleation is not significant; at higher supersaturation levels, primary nucleation rates increase dramatically. This leads to the concept of a metastable zone in which growth dominates, and a labile zone in which primary nucleation dominates. Typical metastable zone widths range between 1-2°C to 30-40°C; inorganic compounds generally have lower widths than organic ones. This is an important concept, and it can be useful to construct such a diagram for a system and then plot the feed, crystallizer and product temperature/composition points.
Crystal growth rates can be measured by laboratory experiments and are also found in the literature, although there is no good summary of available data. Growth correlations are typically found in the form:
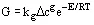
Caution should be exercised when using data of this form, as impurities can have order-of-magnitude effects on growth rates.
Crystals can also join together to form agglomerates: this affects a number of bulk crystal properties including shape, purity, strength, size and packing density. Agglomeration is more usually seen in processes producing small (<50 μm) particles.
Impurities can be incorporated into crystals in a number of ways. Surface impurities are left when residual mother liquor on the surface of the crystals evaporates, leaving behind any dissolved impurities. Inclusions of mother liquor may be formed in crystals, especially at high growth rates. Occlusions are voids formed between individual crystals, usually in agglomerates.
In addition to the growth of crystals, there are also processes where small or large fragments can be attritted or broken off crystals, acting as new nuclei for crystal growth. This reduces the size of large crystals, increases the number of smaller ones, and thus contributes to secondary nucleation. At supersaturation levels below the metastable zone limit, secondary nucleation is the dominant mechanism for formation of new crystals. Another factor to consider is exactly what crystal is forming. For many systems — especially organic ones — more than one structural arrangement is possible, leading to the crystallization of different polymorphs under different conditions. Each polymorph will have a different solubility, stability, etc. The formation of an unstable polymorph is usually undesirable. Unfortunately, the stable polymorph at a particular temperature has the lowest solubility and the slowest growth rates. Solvates can exhibit similar behavior, although the growth unit is different in these cases.
Small crystals (<5 μm), usually formed by precipitation, exhibit an additional effect — size-dependent solubility. The highly curved solid-liquid interface has a higher energy associated with it, and the solubility of very small crystals increases. This leads to a ripening (or ageing) process where smaller crystals held in suspension near the solubility concentration tend to dissolve and larger crystals grow.
Crystals are bounded by their slowest-growing faces. Different faces, especially in organic systems, can have different electronic, structural and chemical characteristics. This can lead to impurities becoming adsorbed to different extents on different faces, causing changes in relative growth rate and hence overall crystal shape or habit. Different growth conditions can also lead to this effect. It is sometimes possible to tailor additives to change crystal habit; more commonly, perhaps, impurities cause unwanted changes.
In solution crystallization, there are a range of crystal sizes present in the vessel. The crystal size distribution can be expressed as number or mass-based, and as a continuous or discrete distribution, usually as the number n of crystals of a particular size L or size range ΔL. From this bulk parameters can be calculated:
(2)
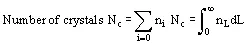
(3)
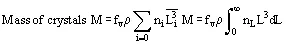
(4)
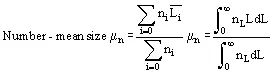
(5)
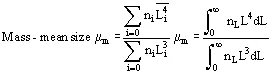
Reference is also commonly made to the moments of distribution:
(6)
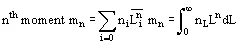
Size distributions are usually summarized in terms of their mass-mean size and coefficient of variation CV. For a Gaussian distribution, CV = standard deviation of size/mean size × 100%, and on a cumulative undersized or oversized plot,
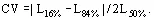
An important concept in the analysis of crystallizer behavior is that of population balance. The number of crystals of a particular size or size range is a balance of the formation and removal rate; for a simple system:
(7)
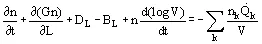
This is most commonly applied to steady-state continuous crystallizers.
For a more complete analysis of population balance and its application to crystallizers, refer to Randolph & Larson (1988) or Nývlt (1992).
Nomenclature
A Constant
B Nucleation rate (kg−1/s)
BLBirth rate of crystals size (m−4/s)
b Supersaturation exponent
c Concentration (kg/kg)
CV Coefficient of variation
DL Death rate of crystals (m−4/s )
E Activation energy (J/mol/K)
fv Volume shape factor
G Growth rate (m/s)
g Supersaturation exponent
J Primary nucleation rate
k Boltzmann's constant (J/K)
kb Secondary nucleation constant
kg Growth rate constant
kn Primary nucleation constant
L Crystal size (m)
M Mass of crystals (kg)
mn nth moment of distribution
MT Slurry density (kg/kg)
Nc Number of crystals
n Number density of crystals (m−4)
![]() Flow rate (m3/s)
Flow rate (m3/s)
R Gas constant (J/mol/K)
S Supersaturation ratio
T Absolute temperature (K)
V Volume of crystallizer (m3)
Ω Molecular volume (m3)
x Mole fraction
β Shape factor
γ Activity coefficient
μ Gibbs free energy (J/mol/K)
μm Mass-mean size (m)
μn Number-mean size (m)
σ Surface tension (J/m2)
ρ Density (kg/m3)