Single Crystals
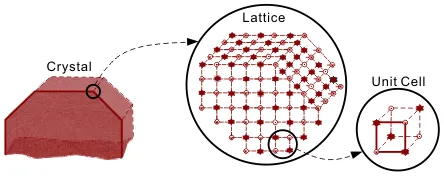
A crystal is a solid regular lattice of atoms, ions or molecules, formed by replicating a unit cell.
These lattices can be categorized by symmetry into a number of crystal systems: regular, tetragonal, orthorhombic, monoclinic, trigonal, triclinic and hexagonal. Over 80% of elements and simple inorganic materials crystallize in the regular or hexagonal systems; complex organics favor orthorhombic and monoclinic systems. The shape or habit of a crystal is defined by the faces of the crystal, which can align in different ways with the crystal lattice. The overall shape of a crystal is defined by the rate at which the various faces grow; the fastest growing faces disappear, leaving the slowest growing faces to dominate. Lattices can also have a range of defects. These can form sites for rapid crystal growth and, in some cases, are the dominant means for crystal growth.
Figure 1.
Figure 2.
Figure 3.