Absorption and Stripping
In absorption (also called gas absorption, gas scrubbing, or gas washing), there is a transfer of one or more species from the gas phase to a liquid solvent. The species transferred to the liquid phase are referred to as solutes or absorbate. Absorption involves no change in the chemical species present in the system. Absorption is used to separate gas mixtures, remove impurities, or recover valuable chemicals. The operation of removing the absorbed solute from the solvent is called stripping. Absorbers are normally used with strippers to permit regeneration (or recovery) and recycling of the absorbent. Since stripping is not perfect, absorbent recycled to the absorber contains species present in the vapor entering the absorber. When water is used as the absorbent, it is normally separated from the solute by distillation rather than stripping

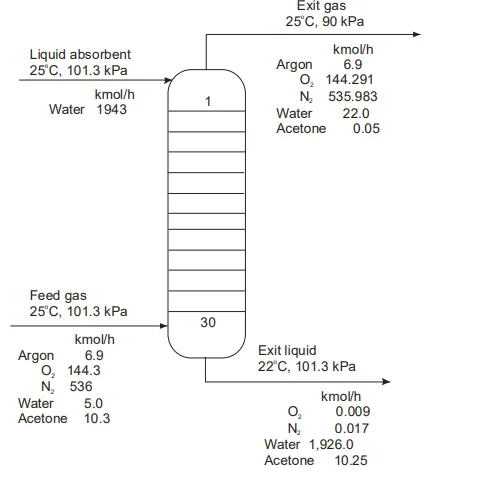
Figure 1: Typical absorption process.
A typical industrial operation for an absorption process is shown in Figure 1 . The feed, which contains air (21% O2, 78% N2, and 1% Ar), water vapor, and acetone vapor, is the gas leaving a dryer where solid cellulose acetate fibers, wet with water and acetone, are dried. Acetone is removed by a 30-tray absorber using water as the absorbent. The percentage of acetone removed from the air stream is
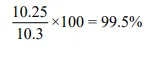
Although the major component absorbed by water is acetone, there are also small amounts of oxygen and nitrogen absorbed by the water. Water is also stripped since more water appears in the exit gas than in the feed gas. The temperature of the exit liquid decreases by 3oC to supply the energy of vaporization needed to strip the water. This energy is greater than the energy of condensation liberated from the absorption of acetone. Three approaches have generally been employed to develop equations used to predict the performance of absorbers and absorption equipment: mass transfer coefficients, graphical solution, and absorption factor. The use of mass transfer coefficient is covered in Chapter 2.2. The graphical solution is simple to use for one or two components and provides explicit graphical presentation of the interrelationships of the variables and parameters in an absorption process. However the graphical technique becomes very tedious when several solutes are present and must be considered. The absorption factor approach can be utilized either for hand or computer calculation. Absorption and stripping are conducted mainly in packed columns and plate columns (trayed tower) as shown in Figure 2.
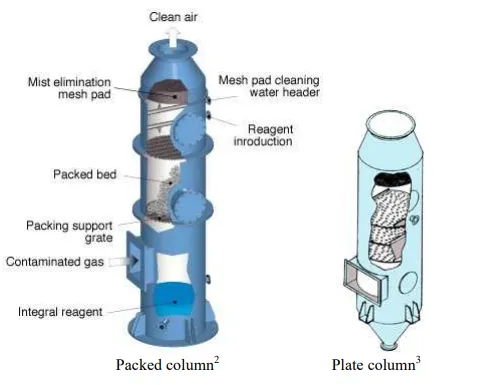
Figure: 2 Equipment for absorption and stripping.
Single-Component Absorption
Most absorption or stripping operations are carried out in counter current flow processes, in which the gas flow is introduced in the bottom of the column and the liquid solvent is introduced in the top of the column. The mathematical analysis for both the packed and plated columns is very similar.
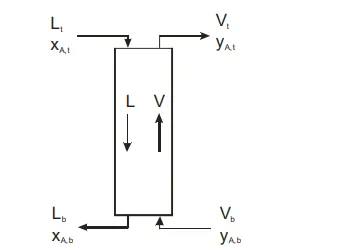
Figure 3: Countercurrent absorption process.