Upgrading and advanced processes
The Refinery make up differs from an upstream plant, in that the overall site is divided up in to process types or 'units'. The refining plant type processes are generally licensed, and a license is required to build and operate one of these. Each license will be the same but scaled to meet the processing capacity in tons per day. A full explanation of these processes is beyond the scope of this book, but a non-exhaustive description is given below.
The following figure gives a more detailed process flow diagram of an actual modern refinery. It shows the extent of treatment that takes place after initial fractional distillation, to improve fuel yield and functional properties, and an explanation of why modern gasoline at the pump contains less than 20% raw gasoline straight from the column. Additional processes may also be included, e.g., for crude pre-treatment to be able to source lower quality crudes with less processing at the production site.

Most of these reactions take place at elevated temperature and pressure over a catalyst such as platinum or rhenium or sometimes iron, and need precise control for optimal throughput. A few process flow diagrams have been included to give an indication of the complexity of these processes compared to the relative simplicity of many upstream processes. Atmospheric distillation is the fractional distillation unit already described. In actual designs, it is combined with vacuum distillation. Raw crude cannot be heated to more than 370-380 °C. It is often called the Crude Oil Distillation Unit (CDU).
Vacuum distillation unit (VDU) further distills the black oils into fuel oils and residual bitumen and coke to avoid overheating the crude and to extract additional valuable product that could be upgraded.
Naphtha hydrotreater: Various sulfur compounds are present in the hydrocarbon mixture and, if burnt with the other carbons, will cause sulfuric emissions. The hydrotreater uses hydrogen to remove some of these compounds. As an example, the hydrodesulfurization (HDS) reaction for ethanethiol can be expressed as:
ethanethiol + hydrogen → ethane + hydrogen sulfide
C2H5SH + H2 → C2H6 + H2S
A catalytic reformer unit is used to convert the naphtha molecules (C5-C12) into higher octane reformate (reformer product). These are mixed with raw gasoline to achieve a higher octane product. The process creates more aromatics (ring formed hydrocarbons) by dehydrocyclization or more complex hydrocarbons with double bonds or side groups by dehydrogenation. These processes release hydrogen which is recovered and can be reused in hydrotreaters or hydrocrackers.
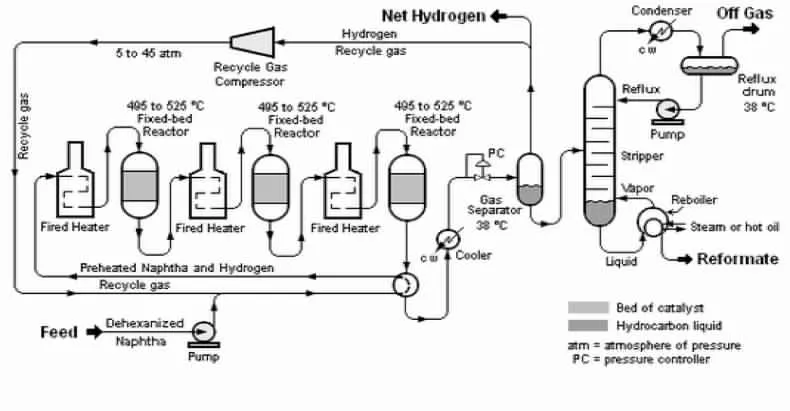
Figure 21. Catalytic reformer
Distillate hydrotreater units desulfurize distillates (such as diesel) after fractional distillation, in the same way as the naphtha hydrotreater.
Fluid catalytic crackers (FCC) units upgrade heavier fractions into lighter, more valuable products. Long chain molecules (high carbon numbers) are split into shorter molecules to achieve more of the high value fuel components. A typical design uses a reactor and a regenerator. A fine powdered porous catalyst with zeolite (silicate and alumina) is fluidized in the hydrocarbon vapor, where a reaction takes place at 535 °C and 0.172 MPa. The catalytic reaction takes place within a few seconds, after which the reformate and catalyst is separated in a cyclone. The spent catalyst then goes back to a regenerator that heats it to 715 °C at 0.241 MPa and releases flue gas. The catalyst powder can then be reused. The reformates go to a distillation column for separation into fractions.
A hydrocracker unit performs essentially the same function as the FCC when more saturated hydrocarbons are desirable in the product. This means alkane carbon chains with single bonds, not double bonds or cyclic rings like aromatics, or more complex molecules. For this, additional hydrogen is needed. The reaction takes place with hydrogen under pressure over a catalyst. The relative market need for diesel, kerosene and gasoline will influence the choice of FCC versus hydrocracker. In the US, with a higher relative volume of gasoline, more FCC capacity is needed, while in Europe and Asia, with higher diesel consumption, more hydrocracking is used.
Visbreaking units upgrade heavy residual oils by thermally cracking them into lower viscosity product that can be blended into lighter, more valuable products. Visbreaking is characterized by its thermal severity, ranging from mild cracking at 425 °C to severe cracking at 500 °C. Depending on the residual oil, as much as 15-25% lighter fractions like diesel, kerosene and gasoline could be obtained. The residue is tar and coke.
The Merox unit treats LPG, kerosene or jet fuel by oxidizing thiols (mercaptans) to organic disulfides. The purpose is to reduce strong odors caused by thiol presence.
Coking units (delayed coking, fluid coker, and flexicoker), like the visbreaker, uses thermal cracking of very heavy residual oils into gasoline and diesel fuel. The residue is green coke, and is further processed to fuel coke or, if too low in sulfur and contaminants, to anode coke for the metallurgic industries.
An alkylation unit produces high-octane components for gasoline blending. The main use is to convert isobutane (C4H10, but arranged differently than n- 84 butane) to alkylates, mainly isooctane or isoheptane by adding an alkyl group such as propene or butene over a strong acid catalyst, such as sulfuric or hydrofluoric acid.
Dimerization is similar to alkylation, but uses a dimer group instead of an alkyl group. For example, butenes can be dimerized into isooctene, which may be hydrogenated to form isooctane.
Isomerization units convert linear molecules to higher-octane branched molecules by rearranging the same atoms arranged in a different way. For example, C4H10 n-butane has the carbon atoms in a chain, while isobutane has a central carbon with one hydrogen and three CH3 groups attached. The isobutane can then be fed to the alkylation unit.
Steam reforming produces hydrogen for the hydrotreaters or hydrocracker. Typical is the steam methane reformer (SMR), where steam reacts with Methane at 425 °C with a nickel catalyst to produce syngas, which is a source for many different reactions:
CH4 + H2O ↔ CO + 3 H2
If more hydrogen is needed, followed by a gas shift reaction with CO:
CO + H2O ↔ CO2 + H2
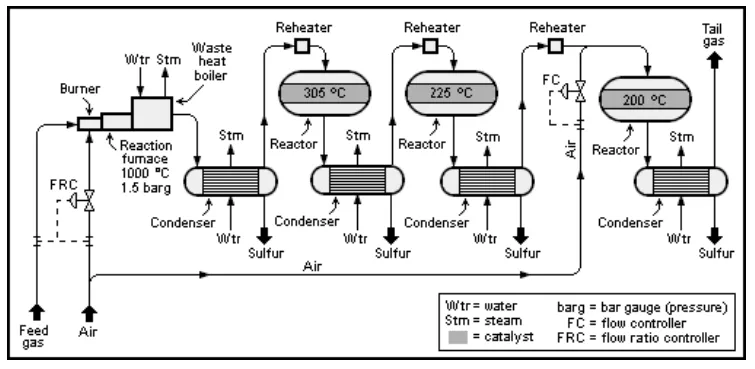
Amine gas treater, Claus unit, and tail gas treatment converts hydrogen sulfide from hydrodesulfurization into elemental sulfur, which is a valuable traded product.

Figure 22. Claus process
The Claus process is the most common with the overall reaction:
2 H2S + O2 → S2 + 2 H2O
The reactor runs at 1,000° C and 0.15 MPa, with three steps: one thermal and two catalytic to improve yield.
Using these processes, a modern refinery can raise the basic gasoline yield depending on crude quality from 10-40% to around to 70%.