Gas processing
Raw natural gas must be processed to meet the trading specifications of pipeline and gas distribution companies. As part of the purification other components such as NGL is produced, and pollutants extracted.
The diagram shows an overview of a typical gas plant. Marketable products are listed in blue and the production process is shown in grey as it is not considered part of the gas plant.

Acid gas removal
Acid gases such as carbon dioxide and hydrogen sulfide form acids when reacting with water, and must be removed to prevent corrosive damage to equipment and pipelines. Hydrogen sulfide is also toxic and total sulfur content is normally regulated.
The main removal process can be based on several principles:
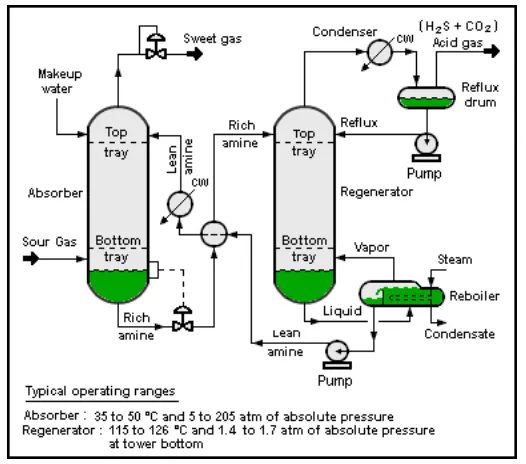
Absorption allows acidic gases to be dissolved in a solvent, to be released by regeneration in a later stage. Amine absorption (as shown on the right) is the most common process for acid gas removal. Monoethanolamine (MEA) dominates for CO2 removal. Solutions with inorganic solvents based on ammonia are under development.

A typical amine gas treating process (as shown in the flow diagram) consists of an absorber unit, a regenerator unit and accessory equipment. In the absorber, a "lean" amine solution absorbs H2S and CO2 from the upflowing sour gas to produce a sweetened gas stream as a product. The "rich" amine solution contains the absorbed acid gases and is routed into the regenerator (a stripper with a reboiler). The stripped overhead gas from the regenerator is concentrated H2S and CO2.
Adsorption relies on the molecules to bind to the surface of certain solids. After a certain time the material must be regenerated to release the gas. Principles used include pressure swing adsorption (PSA), temperature swing adsorption (TSA) and electric swing adsorption (ESA).
Cryogenic removal uses a turbo expander: A gas turbine is driven by the expanding gas which then cools to below the dew point for the gas to be removed. The inlet gas to the compressor is precooled by the acid gas removed. Cryogenic removal is most often used when the content of carbon dioxide is high, typically around 50%
Membrane based removal is based on certain materials that allow the acid gases, but not the hydrocarbons, to diffuse through the membrane. This procedure can be performed alone or in combination with absorption liquid.
Sulfur Unit. The H2S-rich stripped gas stream is then fed to a Claus process – a multistage process with two main sections: A thermal section fires H2S with air or oxygen to produce SO2 and elemental sulfur, which is released when cooled. A catalytic section allows more H2S to react with SO2 with alumina or titanium dioxide (TiO2) to produce water and elemental sulfur (the Claus reaction: 2H2S + SO2 → 3S + 2H2O). The Claus process can recover 95-97% of the sulfur in the feed gases.
A tail gas treatment unit serves to reduce the sulfur content to below 250 ppm, corresponding to a total sulfur recovery of 99.9%. More complex solutions can reduce total sulfur down to 10 ppm. Some important processes include SCOT (Shell Claus offgas treatment) which removes SO2 by combustion with hydrogen over catalysts to produce H2S and water. H2S is recycled to the Claus unit. Other solutions are the Beavon sulfur removal process (BSR), based on amine solvent and catalysts
Dehydration:- Dehydration is either glycol-based scrubbers is based on pressure swing adsorption (PSA). Newer processes also use membranes. (More details will be updated)
Mercury removal
Mercury removal is generally based on molecular sieves. A molecular sieve is a substance containing a material with tiny pores to achieve a large surface area, such as activated carbon. The surface of the material allows certain molecules to bind by surface tension. The molecules can later be extracted and the sieve material regenerated by heating, pressure and/or purging with a carrier gas.
A molecular sieve is commonly cyclic with one active unit and one (or more) units in regeneration.
Nitrogen rejection
Excessive nitrogen is removed by cryogenic distillation and higher concentrations are removed by absorption with lean oil or another special solvent if a smaller fraction is detected. (See acid gas removal for both principles). Cryogenic removal also permits production of helium, if present, as a valuable byproduct.
NGL recovery and treatment
Remaining NGLs are recovered from the gas stream in most modern plants by a cryogenic turbo expander-based process followed by a fractionating process. This process leads the cooled NGLs though distillation columns called de-ethanizer, de-propanizer and de-butanizer, to extract ethane, propane and butane respectively and leave a residual stream of pentane and higher hydrocarbons.
The final step is to remove mercaptans (smelly organic gases, e.g., CH3SH) if present, in a sweetening process based on molecular sieves adsorption or catalytic oxidization such as Merox mercaptan oxidization or Sulfrex, where the main difference is the type of catalyst.
Sales gas specifications
The exact sales gas specification is specified by pipeline operators and distributors. Typical standard sales gas requirements use the following parameters:
Volume is measured in standard cubic meters (scm) defined as 1 m3 at 0 ºC and 101.325 kPa or standard cubic feet (scf) as 1 ft3 at 60 °F (16 °C) and 14.73 PSIA.
Calorific value specifies the total amount of energy per unit generated during combustion of the gas. The value is used to calculate the amount of energy delivered. Several values are listed:
v Gross calorific value or gross heat of combustion is the heat released when a specific quantity of fuel in mixture with air is ignited and the end products have returned to the initial temperature, normally 25 ºC. EU specifications are typically 38.8 MJ (10.8 kWh) ±5% per scm. In the US 1030 BTU ±5% per scf.
v Net calorific value or net heat of combustion is the net heat generated when the water vapor in the gas does not condense (water forms during combustion) and can be 10% lower.
Wobbe index measures the heating effect that a burner is exposed to during combustion. A higher value means a greater thermal load on the burner. Different gases with the same Wobbe index will impose the same load on the burner. An excessively high value is a safety hazard, as it can lead to burner overheating and to excess production of carbon monoxide during combustion.
Calorific value and Wobbe index can be adjusted by blending gas from different sources as well as by addition or removal of nitrogen (N2).
Methane number is a value similar to octane value for gasoline, and is important when the gas is used for internal combustion engines (as CNG).
Hydrogen sulfide and overall sulfur content: Both hydrogen sulfide (H2S) and total sulfur must be reduced. H2S is toxic as well as corrosive for the pipeline, as it forms sulfuric acid (H2SO4) and should be kept as low as possible. Typical maximum values are 5 mg per scm of H2S and total sulfur at 10 mg per scm.
Mercury should be kept below 0.001 ppb (parts-per-billion) which is its detectable limit. The goal is to limit emissions and to prevent damage to equipment and pipelines by mercury amalgamation, which makes aluminum and other metals brittle.
Dew point is a temperature below which some of the hydrocarbons in the gas can condense at pipeline pressure, forming liquid slugs that can damage the pipeline. The gas must also be clear of all water vapor to prevent the formation of methane hydrates within the gas processing plant or within the sales gas transmission pipeline.
Particles and other substances must be free of particulate solids and all liquids to prevent erosion, corrosion or other damage to the pipeline and satisfy limits on carbon dioxide, nitrogen, mercaptans, etc.
Additives: When the natural gas is intended for domestic use, tetrahydrothiophene (THT) is added so that the otherwise odorless natural gas can be detected in the event of a gas leak. The sulfurous-smelling substance added is equal to a sulfur content of 4-7 mg per scm.