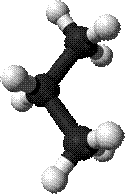Butane vs Propane vs Isobutane - What is Butane?
Liquefied Petroleum Gas
· Discover the real difference between butane vs propane vs isobutane vs LPG. All three gases are consider to be LPG - Liquefied Petroleum Gas.
· In some ways they are almost or exactly the same.
· However, there are some important differences that you need to know.
First, some short summary answers:
Butane vs Propane - Choosing Butane or Propane Gas
· Comparing butane vs propane, the most important differences are their dissimilar boiling point and vapour pressure but both are regarded as LPG – Liquefied Petroleum Gas – and commonly used for cooking, heating, hot water and autogas.
· When considering butane or propane gas, the difference in physical properties determines which gas is best for a particular application.
· Propane has a lower boiling point, at -42°C vs -0.4°C for butane. So, propane will continue to vaporise – turn to gas – even in colder climates, down to -42°C.
· Butane has a lower vapour pressure at a given temperature, being about ¼ that of propane. This lower pressure is advantageous for some propellant applications.
· Butane has a slightly higher energy content by volume while propane energy content is slightly higher by weight. This seeming inconsistency is as a result of the two liquefied gases having a different specific gravity.
What is Butane (n-butane)? Is Butane a Gas?
Ø Butane is a gas when not under pressure and at normal room temperatures. Butane is a flammable hydrocarbon gas that is liquefied through pressurisation. Butane is a gas that also falls under the category of "LPG". It is classified as LPG, along with propane, isobutane and mixtures of these gases.
Ø Butane (n-butane) comes from natural gas processing and oil refining.
Ø Butane is commonly used as a fuel, propellant and refrigerant, as well as a petrochemical feedstock.
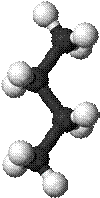
Ø The chemical formula for Butane is C4H10. (Butane molecule model shown)
Ø Butane is supplied to businesses that require Butane, as opposed to propane.
Ø Butane has some specific applications where it has advantages over propane.
n-Butane or n Butane
Both n-Butane or n Butane are just other names for regular butane.
What is Isobutane?
ü Isobutane (i-butane) is an isomer of butane.
ü So, it has the same chemical formula as butane — C4H10 — but has a different arrangement of its atoms, as you can see in the 3-D model images. (Isobutane molecule model shown)
ü As with normal butane, isobutane is a flammable hydrocarbon gas that is liquefied through pressurisation.
ü Isobutane is converted from butane in a process called isomerization.
ü It also has different physical properties from normal butane (n-butane).
ü In addition to being used as a fuel, isobutane is commonly used as a refrigerant and a propellant.
ü Isobutane has very low global warming potential and insignificant ozone depletion potential.
ü However, its main use is in refineries to increase octane of gasoline and make it cleaner burning.
ü It is classified as LPG, along with propane, butane and mixes of these gases.
i-Butane, i Butane or Methylpropane
· i-Butane or i Butane are just other names for isobutane.
· Methylpropane is yet another name for isobutane.
What is Propane Gas?
Ø Propane is a flammable hydrocarbon gas that is liquefied through pressurisation.
Ø It is classified as LPG – Liquefied Petroleum Gas – along with butane, isobutane and mixtures of these gases.
Ø Propane comes from natural gas processing and oil refining.
Ø It is commonly used for heating and cooking.
Ø Propane is the gas that is supplied to virtually all homes and most businesses that purchase LPG in Australia.
Ø LPG is supplied in gas bottles that are either exchanged or refilled on site by LPG tankers.
Ø Large users may utilise bigger LPG storage tanks.
Ø Propane is also frequently used in Autogas, alone or in a propane-butane mix.
Ø LPG goes by a number of names in Australia including LPG, LPG gas, bottled gas, propane, BBQ gas, camping gas and LP gas.
Ø However, no worries, as it’s all the same gas.
Ø The chemical formula for Propane is C3H8. (Propane molecule model shown)
Butane Boiling Point
· The boiling point temperature of butane is -0.4°C.
· This is significantly higher than propane and can be problematic in colder climates.
Propane Boiling Point
· The boiling point temperature of propane is -42°C.
· This boiling point temperature is sufficiently low that vaporisation can be achieved in almost all ambient temperature situations, outside of maybe the polar regions.
Butane or Propane Gas - When are they Liquid or Gaseous?
|
When are Propane & Butane Liquid or Gas? |
||
|
LPG (1atm) |
Liquid |
Vapour (Gas) |
|
Propane |
< -42°C |
≥ -42°C |
|
Butane |
< -0.4°C |
≥ -0.4°C |
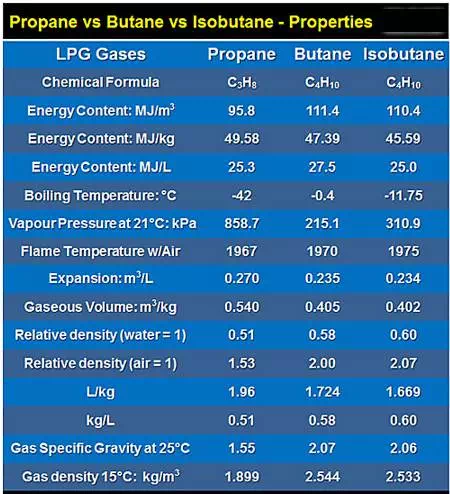
Propane, Butane & Isobutane Properties
· This chart shows some of the physical property differences between the three gases.
· You can refer back to the chart as we explain the importance of the numbers in the following topics…
|
Isobutane vs Butane vs Propane Properties |
|||
|
Gas Properties |
Isobutane |
Butane |
Propane |
|
Chemical Formula |
C4H10 |
C4H10 |
C3H8 |
|
Energy Content: MJ/m3 |
110.4 |
111.4 |
95.8 |
|
Energy Content: MJ/kg |
45.59 |
47.39 |
49.58 |
|
Energy Content: MJ/L |
25.0 |
27.5 |
25.3 |
|
Boiling Temp: Cº |
-11.75 |
-0.4 |
-42 |
|
Pressure @ 21ºC: kPa |
310.9 |
215.1 |
858.7 |
|
Flame Temp: Cº |
1975 |
1970 |
1967 |
|
Expansion: m3/L |
0.234 |
0.235 |
0.270 |
|
Gas Volume: m3/kg |
0.402 |
0.405 |
0.540 |
|
Relative Density: H2O |
0.60 |
0.58 |
0.51 |
|
Relative Density: air |
2.07 |
2.00 |
1.53 |
|
L per kg |
1.669 |
1.724 |
1.96 |
|
kg per L |
0.60 |
0.58 |
0.51 |
|
Specific Gravity @25ºC |
2.06 |
2.07 |
1.55 |
|
Density @ 15ºC: kg/m3 |
2.533 |
2.544 |
1.899 |