Bond Formation and Reaction Energy
Chemistry can be confusing because the generic term energy is thrown around quite a bit. You have probably read, even in these articles, that bond formation releases energy only to later read that energy must be USED to form a bond. What is going on here? The confusion comes down to the difference between energy changes in bond formation/destruction and energy changes as a result of an overall reaction. Let’s take a look at the difference.
Bond Formation and Destruction
The formation of a chemical bond is nothing more than an attraction between two different atoms. So why do atoms become attracted to one another? Well, the answer has to do with protons and electrons.The nucleus of an atom is made up of protons, which have a positive charge, and neutrons (except for hydrogen), which have no charge at all. Circling around the nucleus is a cloud of electrons, which have a negative charge. Now, The type of atom you have is determined by the number of protons. For instance, one proton gives you hydrogen, two helium, six is carbon, and eight is oxygen. In most cases, the protons are balanced by electrons so that the atom has an overall neutral charge. So oxygen also has eight electrons. (We won’t consider isotopes and ions here). Okay, so far so good. If you look at the periodic table, you will see an interesting arrangement of atoms. The reason that the table has such a unique shape is that atoms in the same column behave similarly to each other. As it turns out, this similar behavior has to do with electron configurations.
Electrons behave in certain ways, but to keep things straightforward, we will consider only one property.Eelectrons form orbits around a nucleus. What this means is that electrons aren’t just anywhere in the cloud surrounding a nucleus. Instead, they fall into specific patterns called orbits. As it turns out, each of these orbits (called orbitals) can hold a defined number of electrons. For instance, the first orbit can hold two, the second can hold eight, the third can hold eight, etc. The shape and configuration of orbitals gets very complicated, but for now we just need to know that filling an orbital makes an atom stable. So, the last column on the right of the table represents atoms that are the most stable. Every atom wants to be like the atoms in that last column, but there is a problem. If an atom gives up an electron, then it becomes unstable because the charge from its nucleus is not balanced by the charge from the electrons. These high energy atoms (called ions) don’t hang around for long. The question is, what do they do? The answer is they form pairs with other atoms and they share electrons via a chemical bond. In so doing, all atoms involved in the bond are able to get enough electrons to fill their orbitals, but because they are “sharing,” they don’t run into the charge balance problem that ions have. Let’s look at an example: A hydrogen atom has one electron, which means it is one away from being stable like helium, which has two electrons. Oxygen, which has eight electrons, needs 10 to stable like neon. If oxygen “bonds” to two hydrogen atoms, then it will have 10 electrons because it shares one with each hydrogen atom. By the same measure, each hydrogen atom also gets to share one electron from oxygen, thus reaching the two it needs to be stable. This formation, which is water, is more stable than the form in which oxygen and hydrogen are separate. The more stable something is, the lower its energy will be. So, energy is released when oxygen and hydrogen combine to form water. This is true of all bonds.
Reaction Energy
So we know that forming a bond releases energy. Why then does burning a hydrocarbon produce energy if we are breaking bonds in the process and have to invest energy to do so? The answer to this question is no more complex than adding up the total number of bonds broken versus the total number of bonds formed and taking into account the stability of those bonds (more stable bonds have lower energy than less stable bonds).
In a reaction of methane, we have the following (carbon = black; oxygen = blue, hydrogen = red):
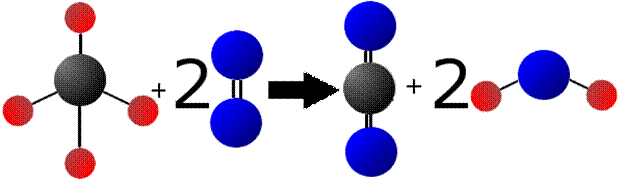
Note that one methane molecule (CH4) combines with one oxygen molecule (O2) to form carbon dioxide (CO2) and 2 water (H2O) molecules. The equation is balanced so that the number of atoms on the left is the same as those on the right. So, there are four hydrogen atoms on the left and four on the right (2 per water multiplied by two water molecules). The same goes for oxygen (four on each side) and carbon (1 on each side).
What is different in the equation above are the bonds. On the left there are eight total bonds (four in methane and two in each of the oxygen molecules). One the right, there are a total of eight as well (4 in carbon dioxide and 2 each in oxygen). So, we didn’t form any extra bonds, but we did form more stable bonds. CO2 is one of the most stable molecules we know of and water, well, we would have so much of it if the bonds were not stable. To break the bonds in methane and oxygen, we had to put some energy in. However, because we get out molecules that are more stable (methane’s instability is evidenced by how easily it burns), we have a net GAIN of energy. This is reaction energy is different from the bond energy, even though they are related.
Conclusion
So, when we talk about energy in reactions and bond, keep in mind that the trend is always toward lower energy. If a bond needs to be broken, then some energy must be added. However, if the end product has more or more stable bonds than the reactant, then there will be a net gain in energy.
The Carbon Cycle
The essential components of hydrocarbons are hydrogen and carbon (big surprise right?). These basic components come from the chemical breakdown of carbon dioxide and water, which takes place in plants. As it turns out, understanding hydrocarbons (at least those that make up fossil fuels) begins with understanding biology and geology. In this article, we look at the biology of carbon. The carbon cycle refers to the natural, biological pathways through which carbon is transferred from non-living substances to living organisms and from one living organism to another. The entire cycle, however, begins with plants.
Plants and Photoautotrophs
Plants are part of a larger group of group of organisms called photoautotrophs. This gigantic term basically means “organisms that make their own food from inorganic (non-living) sources with aide of sunlight.” In other words, photoautotrophs take basic chemicals and, in the presence of light, convert them into more complex molecules that are useful to living things. The basic building blocks of all hydrocarbon molecules come from carbon dioxide (CO2) and water (H2O), which are combined in the process of photosynthesis to create sugars.
Photosynthesis
Photosynthesis is a chemical reaction that converts “reactants” into “products.” In particular, it is a chemical reaction that builds complex molecules from smaller, simpler molecules. Any process that builds a complicated structure requires energy and the energy for photosynthesis comes from the sun. The basic reaction looks like this.
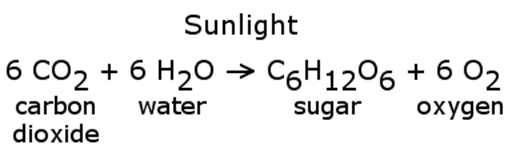
The sugar from this reaction is then used by plant and the animals that eat those plants for both energy and to produce other molecules. At some point, plants and animals die and the sugar contained in their bodies, if subjected to the right conditions, is turned into coal, oil, and natural gas.
Fossil Fuel Formation
The key to turning a dead plant or animal into a fossil and eventually a fossil fuel is anaerobic conditions. That is to say, if there is no oxygen around, then you are well on your way to forming a fossil fuel. Add to this mixture some heat and pressure and you end up with carbon in various forms that include coal, oil, natural gas, graphite, and even diamond.
The basic story is that pressure from multiple layers of sediment leads to an anoxic (oxygen free) environment that allows for decomposition to take place without oxygen (molecular oxygen in the form of O2, that is). When this is combined with heat from the Earth, the carbon in sugar molecules is rearranged to form other compounds. The material that is buried helps to determine what the final product will be. Animal remains tend to form petroleum (crude oil) while plant matter is more likely to form coal and natural gas.
Completing the Cycle – The Slow Carbon Cycle
You may wonder, if carbon is trapped in fossil fuels, how does the carbon cycle make a full circle? Well, first, not all carbon is trapped in fossil fuels. Many plants and animals are decomposed in oxygen-rich environments where microorganisms are able to reclaim the carbon and put it back into the cycle via respiration (the process of breaking down sugar and releasing water and carbon dioxide). Carbon dioxide that is returned this way is often referred to as participating in the “fast carbon cycle.” There is another branch of the cycle, however, that is sometimes called the “slow carbon cycle” because it make take millions of years for carbon to renter the atmosphere.
The second way that carbon reenters the cycle is through volcanoes. Volcanoes emit somewhere between 130 and 380 million metric tons (U.S. tons) of carbon dioxide per year, which is about 100-300 times LESS than the 30 billion tons that humans emit. The third way carbon reenters the cycle is through us burning fossil fuels. When we do this, carbon dioxide and water are release into the atmosphere and taken up by plants for use in photosynthesis. Of course, not all of the carbon is immediately taken back up. Some of it remains in the atmosphere, increasing levels of atmosphere carbon dioxide and contributing to the greenhouse effect.
Fuel from Crude
The primary uses of crude oil to this point have been in the production of fuel. A single barrel of crude oil can produces the following components, which are listed by percent of the barrel they constitute.
- 42% Gasoline
- 22% Diesel
- 9% Jet Fuel
- 5% Fuel Oil
- 4% Liquefied Petroleum Gases
- 18% Other products
Refining
Petroleum refining refers to the process of converting crude oil into useful products. Crude oil is composed of hundreds of different hydrocarbon molecules, which are separated through the process of refining. The process is divided into three basic steps: separation, conversion, and treatment.
Separation
Separation refers to the process of distillation. Crude oil is heated in a furnace so that hydrocarbons can be separated via their boiling point. Inside large towers, heated petroleum vapors are separated into fractions according to weight and boiling point. The lightest fractions, which include gasoline, rise to the top of the tower before they condense back to liquids. The heaviest fractions will settle at the bottom because they condense early.
Conversion
Conversion is simply the process of changing on kind of hydrocarbon into another. Of the, the desired product is gasoline. Cracking is the process of taking heavier, less valuable fractions of crude and converting them into lighter products. Cracking uses heat and pressure to break heavier elements into lighter ones. Alkylation is another common process, which is basically the opposite of cracking. In alkylation, small gaseous byproducts are combined to form larger hydrocarbons.
>Treatment
Treatment is the final process of refining, and includes combining processed products to create various octane levels, vapor pressure properties, and special properties for products used in extreme environments. One common example of treatment is the removal of sulfur from diesel fuel, which is necessary for it to meet clean air guidelines. Treatment is highly technical and is the most time consuming step of refining.
Gasoline
Gasoline is the most popular product derived from petroleum and constitutes the largest fraction of product obtained per barrel of crude oil. The hydrocarbons in gasoline have a chain length of between 4 and 12 carbons. Internal combustion engines burn gasoline in a controlled process called deflagration. Of importance in this process is the timing of combustion, which can be adversely impacted by autoignition of gasoline. This leads to the phenomenon commonly referred to as “engine knock.” In fact, the resistance to autoignition is the largest difference between gasoline and jet fuel, jet fuel being highly resistant to autoignition. A gasoline’s resistance to autoignition is expressed in its octane rating. Octane levels are manipulated by the addition of a particular hydrocarbon called octane. The higher the octane rating of the gasoline, the more the fuel can be compressed. Higher compression means higher temperature and pressure can be achieved inside the engine, which translates to higher power output.
Diesel
Diesel fuel consists of hydrocarbons of a chain length between eight and 21 carbon atoms. Diesel has higher energy content per volume than gasoline. Because they hydrocarbons in diesel are larger, it is less volatile and therefore less prone to explosion, which is one reason it is preferred in military vehicles. Unlike gasoline engines, diesel engines do not rely upon electrically generated sparks to ignite the fuel. Diesel is compressed to high degree along with air, creating high temperatures within the cylinder that lead to combustion. This process makes diesel engines highly efficient, achieving up to 40% better fuel economy than gasoline powered vehicles. Until recently, diesel fuel contained a high degree of sulfur, which contributes to acid rain. Because of their similar distillation points, diesel and sulfur contaminants are removed from crude at the same time during refining. Government regulation now requires that additional steps be taken to remove the sulfur so that diesel fuel is more environmentally friendly. This is part of reason that diesel fuel costs more than gasoline
Heating Oil and Fuel Oil
Fuel oil is one of the “left-over” products of crude refining. It is often less pure than other refined products, containing a broader range of hydrocarbons. Because of its contaminants, fuel oil has a high flash point and is more prone to autoignition. It also produces more pollutants when burned.
Jet Fuel
Jet fuel requires specific characteristics. Namely, it must have a low flammability and it must be able to experience the cold temperatures associated with high altitude without freezing. Jet fuel is based on kerosene, which is slightly heavier than gasoline. Additives help to ensure that it is highly compressible, has a low volatility, and will be free from freezing. Jet fuel comes in three main types:
Jet A
Used only in the United States. Flash point of 38 C (100 F) and autoiginition temperature of 210 C (410 F). This makes jet fuel safer than traditional gasoline.
Jet A-1
Jet A-1 is similar to Jet A, but with a lower freezing point of 47 C.
Jet B
Jet B is designed for use in cold climates. It has a lower autoignition temperature, which makes it more dangerous than Jet A fuels.