The Chemistry of Petroleum Formation
At its base, petroleum is a fossil fuel, which means it is derived from the remains of organic material. In other words, petroleum results from a number of chemical reactions that occur to material that was once alive. In most cases, liquid petroleum was once zooplankton or algae that settled to the bottom of a sea or lake and was then buried under sediment. The sediment ensured that no oxygen was able to reach the decaying organic matter and this set the stage for the formation of oil. In most cases, the organic matter goes through several changes that take thousands or millions of years. As sediment continues to pile up and increase pressure on the organic matter, it is first changed into a waxy solid called kerogen. In fact, this material is currently being mined in many “fracking” processes because it can, through chemical conversion, be made into liquid petroleum and natural gas.
Kerogen is formed in a process called diagenesis, the chemical form of which is outlined in the following diagram.
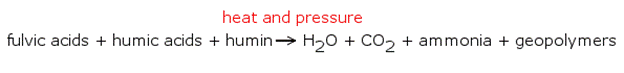
Essentially, heat and pressure break down organic compounds like humin (not human) and various other organic acids, lipids, proteins, and carbohydrates to form long hydrocarbon chains called geopolymers. These geopolymers are the basis of kerogen. Diagenesis is a critical mechanism in the formation of coal and is just the first of several processes necessary to convert solid hydrocarbon to liquid petroleum. The addition of greater heat is necessary to convert kerogen to liquid or gaseous hydrocarbons and the process takes time. The combination of high temperature and pressure is necessary to carry out the endothermic process known as hydrocarbon pyrolysis. It is sometimes referred to as cracking as well.
Hydrocarbon pyrolysis is irreversible, which means that once a liquid hydrocarbon is formed, it is not converted back into solid form. This is why oil deposits can exist below the surface for millions of years unchanged. Liquid hydrocarbons are really just formed by breaking longer chains. It is a general rule in chemistry that the larger a molecule is, the more likely it is to be solid and the smaller a molecule is, the more likely it is to be a liquid or gas. Long hydrocarbon chains are solid, while medium chains (5 – 25 carbons long) are liquid. Smaller chains (less than 5 carbon atoms), tend to be gases. That is why gasoline at 7 or 8 carbons is a liquid while methane, with only one carbon atom, is a gas.
Versions of cracking are used in industry to create everything from charcoal to carbon fiber to biofuels. The process is often used in oil refineries to breakdown the less valuable heating oil molecules (25 carbons per chain on average) into smaller, more valuable 7 and 8 chain molecules that can be sold as gasoline.
The Importance of Oxygen
Oxygen is critical to many processes and its absence is absolutely critical to the formation of hydrocarbons. When oxygen is present, several things can happen. At the surface, when organic material is first laid down, the presence of oxygen means the presence of bacteria that can quickly consume the decaying material before it has a chance to be buried by sediment. This is why most petroleum deposits were once at the bottom of a sea or lake, often one with very low oxygen content, where sediment had time to accumulate before too much decay could occur in the presence of oxygen.
If oxygen is present, besides derailing the early stages of kerogen formation completely, it can also lead to the formation of acids and other molecules rather than strict hydrocarbon. These are usually detrimental to the formation of hydrocarbon and can even reverse formation that has already occurred.
Finally, levels of oxygen that are not high enough to prevent hydrocarbon formation can still be a problem Low levels of oxygen can lead to the buildup of toxic nitrogen oxide compounds as well as sulfuric and sulfurous acids. All these act as contaminants in petroleum, making it more expensive and difficult to refine.
Natural Gas
Natural gas is simply methane and can be associated with oil fields or found in its own deposits. In either case, natural gas can be thought of as the last product of a chain of cracking reactions. Methane is a single carbon atom with four hydrogen atoms. It is the simplest, smallest hydrocarbon and thus cannot be broken down further.
Reservoirs made only of natural gas have occurred in one of two ways. Either the natural gas has leaked from another petroleum deposit that contains other hydrocarbons or all of the hydrocarbons in the deposit have been converted to methane, leaving few if any other hydrocarbons. High temperatures and pressures are necessary for natural gas formation. As a general rule of thumb, the lower the pressure and temperature, the heavier the hydrocarbon will be. Natural gas is only found near the surface if it has escaped from a deeper well.
Methane is also commonly produced by bacteria, making it rather unique among petroleum products (though there are limited instances in which bacteria have been shown to produce things like butane). The bacteria that produce methane are known as methanogens, and can produce methane directly from organic material under anoxic (oxygen free) conditions. These are the bacteria that cause methane production in landfills. Some natural gas may be formed this way during early stages of petroleum formation, but most is likely lost to the atmosphere if there is not a solid layer of sediment to trap it.
The Geology of Petroleum Reserve Formation
Petroleum does not form in reservoirs, but it does collect in them. For a reservoir (just a large accumulation of liquid crude) to form, three conditions must be present. First, there must be petroleum formed deep in the soil. Second, the rocks in which petroleum formation takes place must be porous so that the liquid can move around. Finally, there must be a solid surface above the reservoir that prevents it from being pushed to the surface. If all three of these elements are present, then a reservoir will form. In the diagram below, the kerogen deposit is where all of the chemistry takes place and where all of the liquid petroleum is formed. This is where the heat and pressure work to convert organic matter into liquid hydrocarbon. The kerogen deposit itself is surrounded by porous rock, which is then surrounded by harder, non-porous rock. This is important because the hydrocarbon needs to be able to leak from the kerogen deposit, but not leak so much so as to reach the surface or be able to fall so deep that it cannot be recovered. So, the ideal scenario for the formation of an oil reserve is porous rock with non-porous rock above and below it.
Once the hydrocarbon hits solid rock, it will follow that rock until it reaches a depression. On the surface, this depression would form a lake or pond. Under the surface, the depression remains empty unless the geology is such that petroleum or some other liquid can reach it. The surface of the reservoir is important. It must be solid. The reason it must be solid is that petroleum contains both liquid and gas. As the gas builds, pressure in the reservoir increases and without a solid surface, it would push the petroleum to the surface. In fact, this pressure is the reason that humans have been able to extract petroleum in the past. Only recently, as supplies dwindle, have we been forced to pump the liquid to the surface. Early wells had enough pressure to create the geyser-like explosions depicted in movies.
If a reservoir contains water, and they often do, the water will be found below the petroleum because it is denser. The heaviest petroleum molecules will float on the surface of the water and above them will be lighter molecules typically used in gasoline. At the very top of the reservoir will be natural gas.
If pressures are high in the reservoir, natural gas will be forced into solution. In other words, the natural gas will be dissolved in the liquid petroleum. Even then, however, it will make up the topmost fraction of the liquid. When pressures are low, such as when the well is drilled, the natural gas is the first to escape solution. It is the escape of natural gas that makes explosions a possibility when drilling for oil. Even the smallest spark can ignite methane.