Hydrocarbons
A hydrocarbon is an organic compound composed of two elements, hydrogen and carbon. A large part of the composition of petroleum is made up of hydrocarbons of varying lengths. The smallest hydrocarbon, methane, is composed of a single carbon atom and four hydrogen atoms. However, hydrocarbons can literally consist of hundreds or thousands of individual atoms that are linked together in any number of ways, including chains, circles, and other complex shapes.
Properties
Because the structure of different hydrocarbons can vary so drastically, the properties of each class of molecule vary greatly as well. In order to help categorize the properties of hydrocarbons, they are broken up into several basic types.
- Alkanes – These are referred to as saturated hydrocarbons. Saturated has a specific definition in terms of carbon-based molecules. Carbon can form up to four separate bonds with four separate other atoms. However, it is also possible for carbon to form multiple bonds with a single atom, even another carbon atom. When two carbon atoms in a hydrocarbon are linked together by two or more bonds rather than one, the molecule is termed unsaturated. All alkanes are saturated, which means they only contain single bonds between all carbon atoms. Alkanes are the basis of petroleum fuels and are found in linear and branched forms.
- Unsaturated Hydrocarbons – Those hydrocarbons that have one or more double bonds between carbon atoms are called alkenes. Those with one or more triple bonds between carbon atoms are called alkynes. These are mixed with alkanes in petroleum and contribute more carbon dioxide per pound than do saturated hydrocarbons.
- Cycloalkanes – Any hydrocarbon containing one or more ring structures. These are generally used for the same functions as the non-cyclic alkanes, though they have additional uses in creating certain plastics and in pharmaceutical bases.
- Aromatic Hydrocarbons – This class of molecules has specialized ring structures where bonds between carbon atoms are an intermediate between single and double bonds. Molecules in this class include the industrial solvent benzene.
The structure, hydrogen to carbon ratio, and the length of a particular hydrocarbon determine its properties. In general, small linear hydrocarbons will be gases while medium sized linear hydrocarbons will be liquids. Branched hydrocarbons of intermediate size tend to be waxes with low melting points. Long hydrocarbons tend to be semi-solid or solid. Unsaturated hydrocarbons are more likely to be solid than their saturated counterparts as are cyclic hydrocarbons.
Hydrocarbons in Fuel
Hydrocarbons containing between six and 10 carbon molecules are the top components of most fuels, regardless of whether they are alkanes, alkenes, or cyclic. In general, these molecules are burned to produce energy. Burning hydrocarbons requires oxygen. The hydrocarbon and oxygen combine, in a process called combustion, to produce water, carbon dioxide, and energy. Of course, these molecules are not the only products of the combustion of hydrocarbon. Hydrocarbons that are contaminated with atoms such as sulfur and nitrogen will also produce nitrogen dioxide and sulfur dioxide. Because hydrocarbons are composed purely of carbon and hydrogen, their combustion with oxygen can only produce water as a result of the combination between hydrogen and oxygen and carbon dioxide as a result of the combination of carbon and oxygen. The energy produced by burning a hydrocarbon comes from breaking both carbon-hydrogen and carbon-carbon bonds and recombining them into carbon-oxygen and hydrogen-oxygen bonds. Because an unsaturated hydrocarbon has fewer hydrogen carbon bonds, it has less hydrogen per molecule than a similar unsaturated hydrocarbon and will produce more carbon dioxide. This also means unsaturated hydrocarbons produce less energy when burned than do saturated hydrocarbons. In order to gain the same amount of energy, a greater quantity of unsaturated hydrocarbon must be burned and as a result more carbon dioxide is created in the process. Thus, unsaturated hydrocarbons are less environmentally friendly than saturated hydrocarbons.
Beyond the release of carbon dioxide, burning hydrocarbons also releases other contaminants into the atmosphere. Because refining hydrocarbons is not perfect process, all fuels will contain some level of contaminants. During combustion, sulfur combines with oxygen to produce sulfur dioxide. Sulfur dioxide later combines with hydrogen in the atmosphere to produce the weak sulfurous acid as well as the strong sulfuric acid. Both of these contribute to acid rain. In addition to sulfur, nitrogen is also a common contaminant in hydrocarbons. Nitrogen dioxide can react with hydrogen in the atmosphere to produce nitric acid, which also contributes to acid rain.
Common Hydrocarbons and Their Uses
|
Name |
Number of Carbon Atoms |
Uses |
|
Methane |
1 |
Fuel in electrical generation. Produces least about of carbon dioxide. |
|
Ethane |
2 |
Used in the production of ethylene, which is utilized in various chemical applications. |
|
Propane |
3 |
Generally used for heating and cooking |
|
Butane |
4 |
Generally used in lighters and in aerosol cans |
|
Pentane |
5 |
Can be used as solvents in the laboratory and in the production of polystyrene. |
|
Hexane |
6 |
Used to produce in glue for shoes, leather products, and in roofing |
|
Heptane |
7 |
The major component of gasoline |
|
Octane |
8 |
An additive to gasoline that reduces knock, particularly in its branched forms |
|
Nonane |
9 |
The component of fuel, particularly diesel |
|
Decane |
10 |
A component of gasoline, but generally more important in jet fuel and diesel |
Hydrocarbons longer than 10 carbon atoms in length are generally broken down
through the process known as “cracking” to yield molecules with lengths of 10
atoms or less.
Aromatic Hydrocarbons
Aromatic hydrocarbons, also called arenes, are a unique class of carbon molecules in which carbon atoms are connected by alternating double and single bonds. The traditional view of such a bond character is diagramed as follows:

The above structure, however, does not aptly describe the character of the bonds between the carbon molecules because the illustrated double bounds are more flexible than standard double bonds while the single bonds are less flexible than single carbon-carbon bonds in other molecules. It is also true that every bond in an aromatic hydrocarbon is the same length, rather than the “single” bonds being longer and the “double” bonds being shorter as would be expected. These properties have led to the term aromaticity, which describes a bond that is intermediate between single and double or a 1.5 bond. Thus, the structure of an aromatic bond is more like the following diagram.
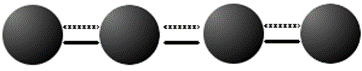
The above diagrams simply show the bond structure, the other important component of an aromatic hydrocarbon is that it is ALWAYS in a ring. This is because a ring is the only way for bonds to be shared equally between carbon atoms. Thus, the actual structure of an aromatic hydrocarbon is best demonstrated by benzene, the type molecule for the class.
Benzene (C6H6)
The structure of benzene can be drawn in two ways. In the first, the double bond character is explicitly drawn. In the shorthand version, a circle is simply drawn inside the ring to demonstrate the structure. Each carbon atom in benzene has a single hydrogen attached to it. In the diagram, the hydrogen have been omitted for clarity.
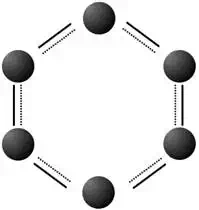
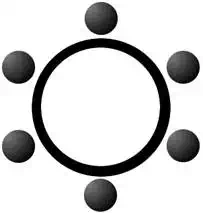
Basic Properties
Like other hydrocarbons, benzene is a natural component of petroleum. It is a colorless, flammable, sweet-smelling liquid at room temperature and is a component of most gasoline mixes as it has a high octane number. Benzene is also highly carcinogenic and is well-known to cause bone marrow failure and bone cancer. Of course, its carcinogencity was not well known when it was being used as an additive in after shave and other cosmetics due to its “pleasant aroma.”
Cancer
What makes benzene carcinogenic is its interaction with DNA. It breaks the bonds between subunits of DNA in the body, which in turn causes cells to either die or reproduce without normal controls (cancer). It is general consensus that no amount of benzene is safe, but this has not stopped its use in many industrial and laboratory applications where is properties have yet to be supplanted by another substance. Toluene is used where possible as a less carcinogenic and less toxic alternative to benzene. Currently, the largest exposures to benzene occur through smoking as it is a byproduct of burning the tar found in cigarettes. The second largest exposure is in gasoline service stations, which, when combined with industrial emissions and the emissions from car exhaust, accounts for 20% of all atmospheric benzene.
Uses
The largest use of benzene (50%) is in the product of styrene and polystyren plastics. It is also converted to a molecule known as cyclohexane, which is important in the production of Nylon. About 15% of benzene is used to produce cyclohexane. Smaller amounts are used in everything from pesticides to rubber to pharmaceuticals.
Other Arenes
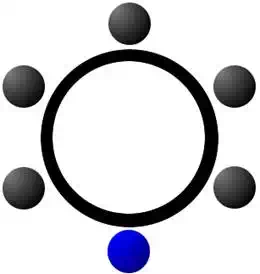
In general, benzene is the only stable aromatic hydrocarbon. Rings with fewer or more carbon atoms are not stable and are of less importance than benzene. In some cases, the substitution of a carbon atom with another element, such as nitrogen, can produce an aromatic structure as well. These are, of course, no longer hydrocarbons, but they do have important applications and can be constructed from aromatic hydrocarbons. Pyridine is the most commonly known aromatic that contains an atom other than carbon. In this case, nitrogen replaced a single carbon atom to produce an aromatic molecule with the formula C5H5N. Pyridine is a common organic molecule that is found in vitamins, various pharmaceuticals, and acts as a precursor to a number of other biologic compounds. (Blue represents nitrogen in this diagram).
Polycyclic Aromatic Hydrocarbons (PAHs)
This term is applied to any hydrocarbon that has two or more aromtaic rings. These molecules are highly lipophilic, which means they have a proclivity for oils and fats, and are also highly carcinogenic. This combination means that these carcinogens enter the body and they do not leave again because they become absorbed into fat molecules. The burning of coal from coal-fired power plants is the main source of PAHs in the atmopshere. PAHs are also found in meats cooked at high temperatures.