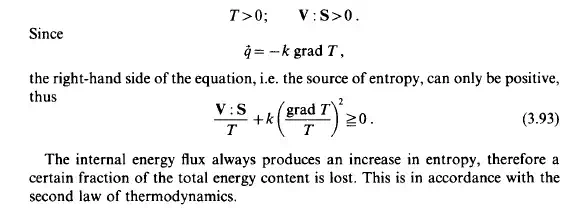
The balance of entropy
The entropy is a thermodynamic variable of state; an extensive quantity. It cannot be experienced directly; as a derived variable entropy represents a measure of the irreversibility of a change of state. Irreversible changes of state necessarily involve currents in the system. The increase in entropy during an irreversible process must be related to these currents.
Entropy is not a quantity which is conserved. There is no axiom to determine the rate of change of entropy during an irreversible process. Thus we can only derive the balance of entropy equation by starting from the internal energy equation.

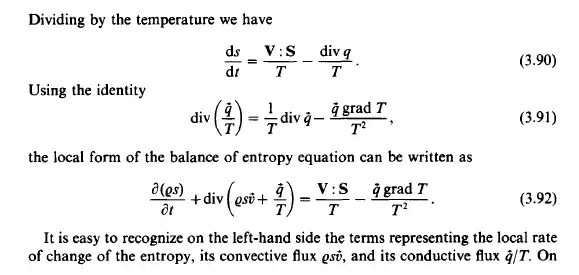
the right-hand side of the equation terms representing the entropy sources are found. The inclusion of the coductive flux term in the entropy equation shows that entropy is always exchanged simultaneously with the internal energy. Considering the source terms, it is obvious that the absolute temperature is always positive, while the dissipating mechanical power is also positive, i.e.