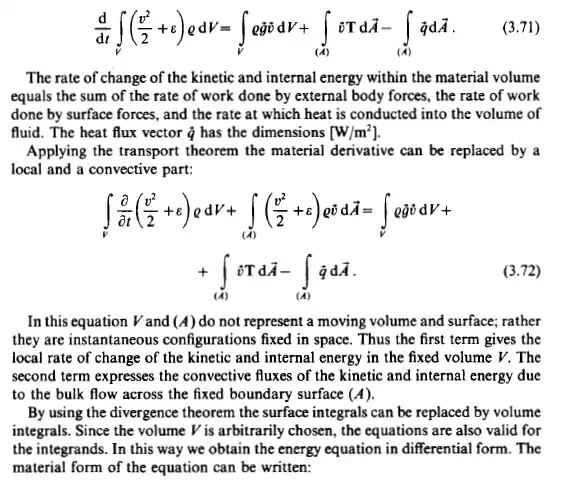
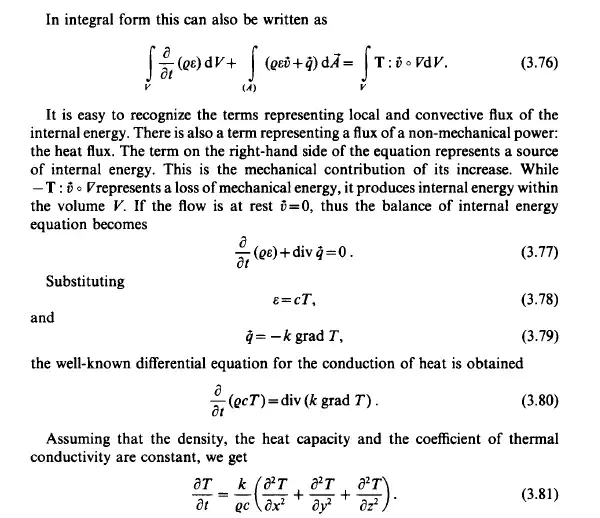
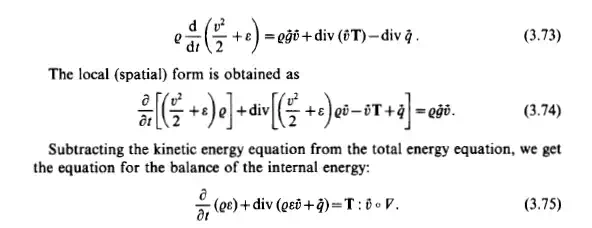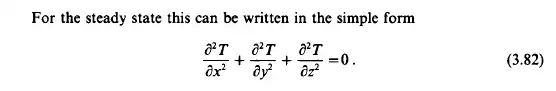The principle of conservation of energy
Any material system is characterized by its energy content. Energy occupies a position of distinction amongst the other extensive variables. Whatever interaction
occurs between a material system and its surroundings, the transfer of energy invariably accompanies the transport of other extensive variables. Certain interactions
are accompanied the transport of a certain type of energy. Kinetic energy increases or decreases due to mechanical interactions; a change in internal energy accompanies thermal interactions. But the fundamental axiom is the principle of conservation of energy which applies to closed systems only. The energy content of an open system may change depending on the action of its surroundings. Consider a system in mechanical and thermal interaction with its surroundings. In this case the change in the total energy
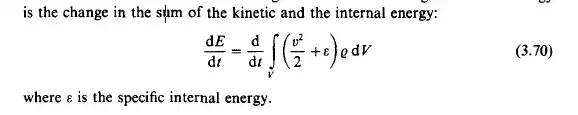
The action of the surroundings may take the form of mechanical work and/or The application of the principle of conservation of energy to this open system heat.
leads to the equation