Surface Renewal Theory
For the mass transfer in liquid phase, Danckwert (1951) modified the Higbie’s penetration theory. He stated that a portion of the mass transfer surface is replaced with a new surface by the motion of eddies near the surface and proposed the following assumptions: 1) The liquid elements at the interface are being randomly swapped by fresh elements from bulk 2) At any moment, each of the liquid elements at the surface has the same probability of being substituted by fresh element 3) Unsteady state mass transfer takes place to an element during its stay at the interface.

Boundary Layer Theory
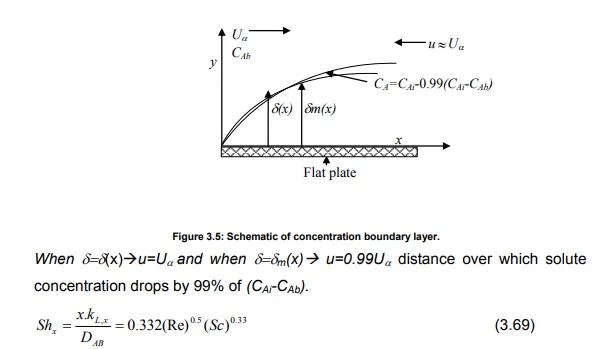
Boundary layer theory takes into account the hydrodynamics/flow field that characterizes a system and gives a realistic picture of the way mass transfer at a phase boundary. A schematic of concentration boundary layer is shown in Figure 3.5.
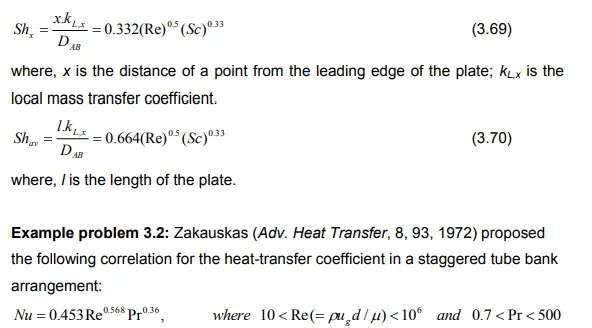
Estimate the mass-transfer coefficient by using the mass and heat transfer analogy if to be expected for evaporation of n-propyl alcohol into carbon dioxide for the same geometrical arrangement of tube diameter (d) of 38 mm when the carbon dioxide flows at a maximum velocity (ug) of 10 m/s at 300 K and 1 atm. Properties of dilute mixtures of propyl alcohol in carbon dioxide at 300 K and 1 atm are: Molecular weight (M) = 44 gm/mole, density (ρ) = 1.8 kg/m3 , Viscosity (μ) = 1.49×10-5 kg/m.s, diffusivity (DAB) = 7.6×10-6 m 2 /s and universal gas