
Biochemical Sedimentary
Rocks The Earth System involves many interactions between living organisms and the physical planet. Numerous organisms have evolved the ability to extract dissolved ions from seawater to make solid shells. When the organisms die, the solid material in their shells survives. This material, when lithified, comprises biochemical sedimentary rock. Geologists recognize several different types of biochemical sedimentary rocks, which we now describe.
Limestone (biochemical)

A snorkeler gliding above a reef sees an incredibly diverse community of coral and algae, around which creatures such as clams, oysters, snails (gastropods), and lampshells (brachiopods) live, and above which plankton float (a figure above). Though they look so different from each other, many of these organisms share an important characteristic: they make solid shells of calcium carbonate (CaCO3). The CaCO3 crystallizes either as calcite or aragonite. (These minerals have the same composition, but different crystal structures.) When the organisms die, the shells remain and may accumulate.
Rocks formed dominantly from this material are the biochemical version of limestone. Since the principal compound making up limestone is CaCO3, geologists refer to limestone as a type of carbonate rock. Limestone comes in a variety of textures, because the material that forms it accumulates in a variety of ways. For example, limestone can originate from reef builders (such as coral) that grew in place, from shell debris that was broken up and transported, from carbonate mud, or from plankton shells that settled like snow out of water. Because of this variety, geologists distinguish among fossiliferous limestone, consisting of visible fossil shells or shell fragments (b figure above); micrite, consisting of very fine carbonate mud; and chalk, consisting of plankton shells. Experts recognize many other types as well. Typically, limestone is a massive light-gray to darkbluish-gray rock that breaks into chunky blocks it doesn't look much like a pile of shell fragments (c figure above). That’s because several processes change the texture of the rock over time. For example, water passing through the rock not only precipitates cement but also dissolves some carbonate grains and causes new ones to grow.
Chert (biochemical).

If you walk beneath the northern end of the Golden Gate Bridge in California, you will find outcrops of reddish, almost porcelain-like rock occurring in 3- to 15-cm-thick layers (a figure above). Hit it with a hammer, and the rock would crack to form smooth, spoon-shaped (conchoidal) fractures. Geologists call this rock biochemical chert; it’s made from cryptocrystalline quartz (crypto is Greek for hidden), meaning quartz grains that are too small to be seen without the extreme magnification of an electron microscope. The chert beneath the Golden Gate Bridge formed from the shells of silica-secreting plankton that accumulated on the sea floor. Gradually, after burial, the shells dissolved, forming a silica-rich gel. Chert then formed when this gel solidified.
Organic Sedimentary Rocks
We've seen how the mineral shells of organisms (CaCO3 or SiO2) can accumulate and lithify to become biochemical sedimentary rocks. What happens to the “guts” of the organisms the cellulose, fat, carbohydrate, protein, and other organic compounds that make up living matter? Commonly, this organic debris gets eaten by other organisms or decays at the Earth’s surface. But in some environments, the organic debris settles along with other sediment and eventually gets buried. When lithified, organic-rich sediment becomes organic sedimentary rock. Since the dawn of the industrial revolution in the early 19th century, coal, one type of organic sedimentary rock, has provided the fuel of modern industry and transportation, for the organic chemicals in the rock yield energy when burned. Coal is a black, combustible rock consisting of over 50 to 90% carbon. The remainder consists of oxygen, nitrogen, hydrogen, sulphur, silica, and minor amounts of other elements. Typically, the carbon in coal occurs in large, complex organic molecules made of many rings note that the carbon does not occur in CaCO3. Coal forms when plant remains have been buried deeply enough and long enough for the material to become compacted and to lose significant amounts of volatiles (hydrogen, water, CO2, and ammonia); as the volatiles seep away, a concentration of carbon remains (b figure above).
Chemical Sedimentary Rocks
The colourful terraces, or mounds, that grow around the vents of hot-water springs; the immense layers of salt that underlie the floor of the Mediterranean Sea; the smooth, sharp point of an ancient arrowhead these materials all have something in common. They all consist of rock formed primarily by the precipitation of minerals from water solutions. We call such rocks chemical sedimentary rocks. They typically have a crystalline texture, partly formed during their original precipitation and partly when, at a later time, new crystals grow at the expense of old ones through a process called recrystallization. In some examples, crystals are coarse. In others, they are too small to see. Geologists distinguish among many types of chemical sedimentary rocks, primarily on the basis of composition.
Evaporites: the products of salt-water evaporation.
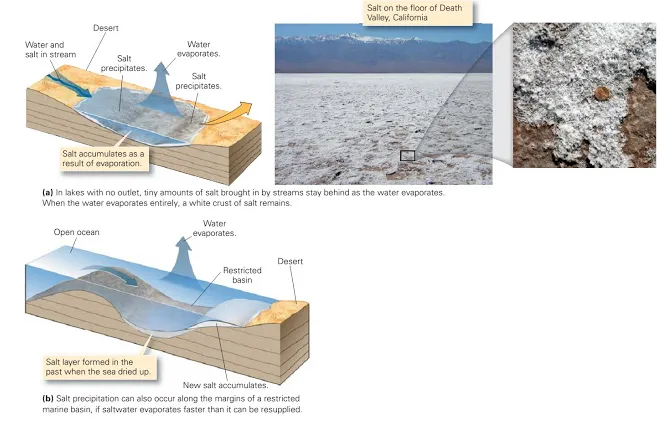
In 1965, two daredevil drivers in jet-powered cars battled to be the first to set the land speed record of 600 mph. On November 7, Art Arfons, in the Green Monster, peaked at 576.127 mph; but eight days later Craig Breedlove, driving the Spirit of America, reached 600.601 mph. Travelling at such speeds, a driver must maintain an absolutely straight line; any turn will catapult the vehicle out of control. Thus, high-speed trials take place on extremely long and flat racecourses. Not many places can provide such conditions the Bonneville Salt Flats of Utah do. The salt flats formed when an ancient salt lake evaporated. Under the heat of the Sun, the water turned to vapour and drifted up into the atmosphere, but the salt that had been dissolved in the water stayed behind. Salt precipitation occurs where salt-water becomes supersaturated, meaning that it has exceeded its capacity to contain more dissolved ions. In supersaturated salt-water, ions bond to form solid grains that either settle out of the water or grow on the floor of the water body. Supersaturated salt-water develops where evaporation removes water from a water body faster than the rate at which new water enters. This process takes place in desert lakes and along the margins of restricted seas (figure above). For thick deposits of salt to form, large volumes of water must evaporate. Because salt deposits form as a consequence of evaporation, geologists refer to them as evaporites. The specific type of salt minerals comprising an evaporite depends on the amount of evaporation. When 80% of the water evaporates, gypsum forms; and when 90% of the water evaporates, halite precipitates.
Travertine (chemical limestone).
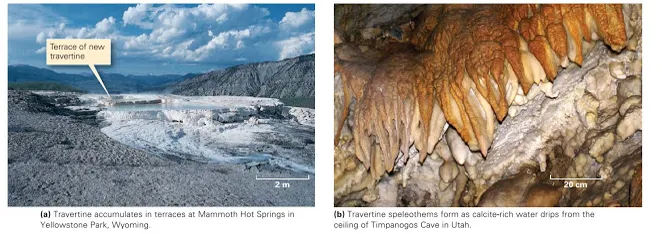
Travertine is a rock composed of crystalline calcium carbonate (CaCO3) formed by chemical precipitation from groundwater that has seeped out at the ground surface either in hot- or cold-water springs, or on the walls of caves. What causes this precipitation? It happens, in part, when the groundwater degasses, meaning that some of the carbon dioxide that had been dissolved in the groundwater bubbles out of solution, for removal of carbon dioxide encourages the precipitation of carbonate. Precipitation also occurs when water evaporates, thereby increasing the concentration of carbonate. Various kinds of microbes live in the environments in which travertine accumulates, so biologic activity may also contribute to the precipitation process. Travertine produced at springs forms terraces and mounds that are meters or even hundreds of meters thick, such as those at Mammoth Hot Springs (a in figure above). Travertine also grows on the walls of caves where groundwater seeps out (b in figure above). In cave settings, travertine builds up beautiful and complex growth forms called speleothems.
Dolostone.
Another carbonate rock, dolostone, differs from limestone in that it contains the mineral dolomite (CaMg[CO3]2), which contains equal amounts of calcium and magnesium. Where does the magnesium come from? Most dolostone forms by a chemical reaction between solid calcite and magnesium-bearing groundwater. Much of the dolostone you may find in an outcrop actually originated as limestone but later changed into dolostone as dolomite crystals replaced calcite. This change may take place beneath lagoons along a shore soon after the limestone formed, or a long time later, after the limestone has been buried deeply.
Chert (replacement).

A tribe of Native Americans, the Onondaga, once lived off the land in eastern New York State. Here, outcrops of limestone contain layers or nodules (lenses or lumps) of a black chert (a in figure above). Because of the way it breaks, the tribe’s artisans could fashion sharpedged tools (arrowheads and scrapers) from this chert, so the Onondaga collected it for their own toolmaking industry and for use in trade with other people. Unlike the deep sea (biochemical) chert described earlier, the chert collected by the Onondaga formed when cryptocrystalline quartz gradually replaced calcite crystals within a body of limestone long after the limestone was deposited; geologists call such material “replacement chert.”
Chert comes in many colours (black, white, red, brown, green, gray), depending on the impurities it contains. Petrified wood is chert that forms when silica-rich sediment, such as ash from a volcanic eruption, buries a forest. The silica dissolves in groundwater, and then later precipitates as cryptocrystalline quartz within wood, gradually replacing the wood’s cellulose. The chert deposit retains the shape of the wood and the growth rings within it. Some chert, known as agate, precipitates in concentric rings inside hollows in a rock and ends up with a striped appearance, caused by variations in the content of impurities incorporated in the chert (b in figure above).