Magnetism of rocks and minerals
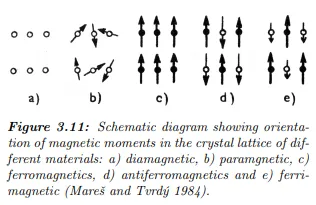
The overall magnetization of the rocks is a vector sum of induced magnetization (the magnetization present only if an external field is applied, ceases, when the external field is removed) and natural remanent magnetization (present even without the external magnetic field). For example, effusive rocks have have the remanent magnetization often much stronger than the induced one. According to their behaviour when placed into an external magnetic field, the materials could be divided into two main groups – diamagnetic and paramagnetic (Fig. 3.11). Diamagnetic material is dominated by atoms with orbital electrons oriented to oppose

the external field – the susceptibility is negative (see Tab.1.1). Diamagnetic materials are graphite, quartz, feldspar, marble, salt, etc. Atoms of paramagnetic materials have non-zero moments without the presence of external field and magnetic susceptibility of such materials is positive.
The direction of magnetization of individual atoms is randomly oriented and their vector sum is non-zero, but weak. In presence of external field, the magnetic atom slightly aligns forming a weak magnetization – an induced magnetization. When the external field is removed, the magnetization ceases. The magnetic effect of diamagnetic and most paramagnetic substances is weak. Certain paramagnetic materials (iron, nickel, cobalt) could have such strong magnetic interactions that the magnetic moments in large regions – domains – align.
This effect is called ferromagnetism and is about 106 times the effect of diamagnetism and ferromagnetism. The ferromagnetism decreases with increasing temperature and ceases when temperature exceeds the Curie point. Some materials have domains further divided into subdomains with opposite orientation and the overall magnetic moment nearly cancels. These materials are called antiferromagnetic and their susceptibility is low. The common example is hematite.
The last group have subdomains also aligned in oppositions, however their net magnetic moment is non-zero. This could be either due to the fact that one orientation of subdomains have weaker moment or that there is less domain with one of orientations. Such substances are called ferrimagnetic.
Examples of the first type are magnetite, titanomagentite, oxides of iron and of iron and titanium. The second group is represented by pyrrhotite. The induced magnetization is directly proportional to the susceptibility and concentration of magnetic minerals present in the rocks. The orientation is, naturally, the same as that of the external field (geomagentic field in our case). However, the measured magnetization is not always of this direction.