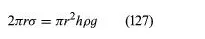
Surface tension of liquids
Of the many hydrostatic phenomena in which the surface tension of liquids plays a role, the most significant is probably capillarity. Consider what happens when a tube of narrow bore, often called a capillary tube, is dipped into a liquid. If the liquid “wets” the tube (with zero contact angle), the liquid surface inside the tube forms a concave meniscus, which is a virtually spherical surface having the same radius, r, as the inside of the tube. The tube experiences a downward force of magnitude 2πrdσ, where σ is the surface tension of the liquid, and the liquid experiences a reaction of equal magnitude that lifts the meniscus through a height h such that
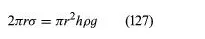
—i.e., until the upward force for which surface tension is responsible is balanced by the weight of the column of liquid that has been lifted. If the liquid does not wet the tube, the meniscus is convex and depressed through the same distance h (see Figure 3). A simple method for determining surface tension involves the measurement of h in one or the other of these situations and the use of equation (127) thereafter.
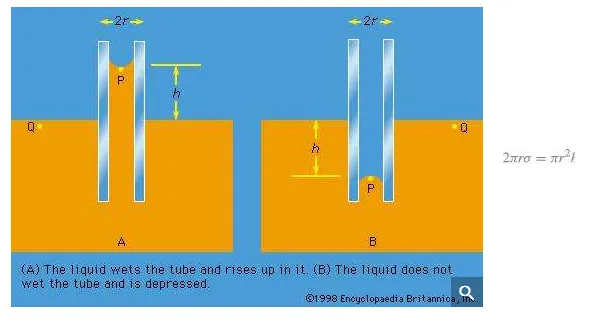
It follows from equations (124) and (127) that the pressure at a point P just below the meniscus differs from the pressure at Q by an amount
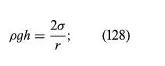
it is less than the pressure at Q in the case to which Figure 3A refers and greater than the pressure at Q in the other case. Since the pressure at Q is just the atmospheric pressure, it is equal to the pressure at a point immediately above the meniscus. Hence, in both instances there is a pressure difference of 2σ/r between the two sides of the curved meniscus, and in both the higher pressure is on the inner side of the curve. Such a pressure difference is a requirement of equilibrium wherever a liquid surface is curved. If the surface is curved but not spherical, the pressure difference is
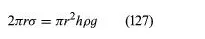
The diagrams in Figure 3 were drawn to represent cross sections through cylindrical tubes, but they might equally well represent two vertical parallel plates that are partly submerged in the liquid a small distance apart. Consideration of how the pressure varies with height shows that over the range of height h the plates experience a greater pressure on their outer surfaces than on their inner surfaces; this is true whether the liquid wets both plates or not. It is a matter of observation that small objects floating near one another on the surface of a liquid tend to move toward one another, and it is the pressure difference just referred to that makes them behave in this way.
One other problem having to do with surface tension will be considered here. The diagrams in Figure 4 show stages in the growth of a liquid drop on the end of a tube which the liquid is supposed to wet. In passing from stage A to stage B, by which time the drop is roughly hemispheric in shape, the radius of curvature of the drop diminishes; and it follows from (128) that, to bring about this growth, one must slowly increase the pressure of the liquid inside the tube. If the pressure could be held steady at the value corresponding to B, the drop would then become unstable, because any further growth (e.g., to the more or less spherical shape indicated in Figure 4C) would involve an increase in radius of curvature. The applied pressure would then exceed that required to hold the drop in equilibrium, and the drop would necessarily grow bigger still. In practice, however, it is easier to control the rate of flow of water through the tube, and hence the rate of growth of the drop, than it is to control the pressure. If the rate of flow is very small, drops will form the nonspherical shapes suggested by Figure 4D before they detach themselves and fall. It is not an easy matter to analyze the shape of a drop on the point of detachment, and there is no simple formula for the volume of the drop after it is detached.