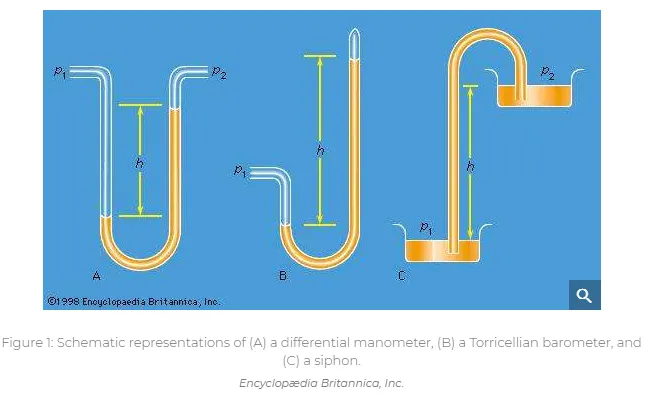
Differential manometers
Instruments for comparing pressures are called differential manometers, and the simplest such instrument is a U-tube containing liquid, as shown in Figure 1A. The two pressures of interest, p1 and p2, are transmitted to the two ends of the liquid column through an inert gas—the density of which is negligible by comparison with the liquid density, ρ—and the difference of height, h, of the two menisci is measured. It is a consequence of (124) that

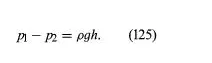
A barometer for measuring the pressure of the atmosphere in absolute terms is simply a manometer in which p2 is made zero, or as close to zero as is feasible. The barometer invented in the 17th century by the Italian physicist and mathematician Evangelista Torricelli, and still in use today, is a U-tube that is sealed at one end (see Figure 1B). It may be filled with liquid, with the sealed end downward, and then inverted. On inversion, a negative pressure may momentarily develop at the top of the liquid column if the column is long enough; however, cavitation normally occurs there and the column falls away from the sealed end of the tube, as shown in the figure. Between the two exists what Torricelli thought of as a vacuum, though it may be very far from that condition if the barometer has been filled without scrupulous precautions to ensure that all dissolved or adsorbed gases, which would otherwise collect in this space, have first been removed. Even if no contaminating gas is present, the Torricellian vacuum always contains the vapour of the liquid, and this exerts a pressure which may be small but is never quite zero. The liquid conventionally used in a Torricelli barometer is of course mercury, which has a low vapour pressure and a high density. The high density means that h is only about 760 millimetres; if water were used, it would have to be about 10 metres instead.
Figure 1C illustrates the principle of the siphon. The top container is open to the atmosphere, and the pressure in it, p2, is therefore atmospheric. To balance this and the weight of the liquid column in between, the pressure p1 in the bottom container ought to be greater by ρgh. If the bottom container is also open to the atmosphere, then equilibrium is clearly impossible; the weight of the liquid column prevails and causes the liquid to flow downward. The siphon operates only as long as the column is continuous; it fails if a bubble of gas collects in the tube or if cavitation occurs. Cavitation therefore limits the level differences over which siphons can be used, and it also limits (to about 10 metres) the depth of wells from which water can be pumped using suction alone.