Capillarity
If a thin tube, open at the both ends, is inserted vertically in to a liquid, which wets the tube, the liquid will rise in the tube (fig : L -3.4). If the liquid does not wet the tube it will be depressed below the level of free surface outside. Such a phenomenon of rise or fall of the liquid surface relative to the adjacent level of the fluid is called capillarity. If is the angle of contact between liquid and solid, d is the tube diameter, we can determine the capillary rise or depression, h by equating force balance in the z-direction (shown in Fig : L-3.5), taking into account surface tension, gravity and pressure. Since the column of fluid is at rest, the sum of all of forces acting on the fluid column is zero.
The pressure acting on the top curved interface in the tube is atmospheric, the pressure acting on the bottom of the liquid column is at atmospheric pressure because the lines of constant pressure in a liquid at rest are horizontal and the tube is open.

Typical values of capillary rise are
· Capillary rise is approximately 4.5 mm for water in a glass tube of 5 mm diameter.
· Capillary depression is approximately - 1.5 mm (depression) for mercury in the same tube.
Capillary action causes a serious source of error in reading the levels of the liquid in small pressure measuring tubes. Therefore the diameter of the measuring tubes should be large enough so that errors due to the capillary rise should be very less. Besides this, capillary action causes the movement of liquids to penetrate cracks even when there is no significant pressure difference acting to move the fluids in to the cracks.
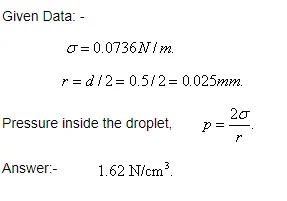
In figure (Fig : L - 3.6), a two-dimensional model for the capillary rise of a liquid in a crack width, b, is illustrated. The height of the capillary rise can also be computed by equating force balance as explained in the previous section.
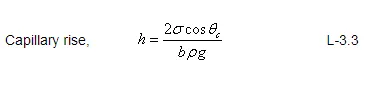
Example
Find the pressure inside a water droplet having diameter of 0.5 mm at 20 0 C if the outside pressure is 1.03N/cm 2 and the surface tension of water at that temperature is 0.0736 N/m.