Gibbs Free Energy and Other Thermodynamic Functions
Dexter Perkins (University of North Dakota), Andrea Koziol (University of Dayton), John Brady (Smith College)

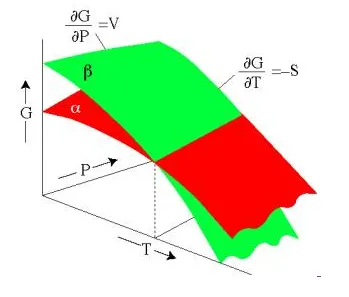
Comparing the Gibbs Free Energy of two minerals, α and β, both with the same composition. The mineral with the lowest Energy is stable. Here we see that α is stable under some P-T conditions, and β is stable under different conditions.
Gibbs Free Energies of Phases
All phases, whether mineralogical or not, have an associated Gibbs Free Energy of Formation value abbreviated ΔGf. The ΔGf value describes the amount of energy that is released or consumed when a phase is created from other phases.
Consider, for example, enstatite (MgSiO3). The Gibbs Free Energy of Formation for enstatite from pure elements (Mg, Si and O) = ΔGf (enstatite, elements) is about -1,460.9 J/mole at room temperature and pressure. The Gibbs Free Energy of Formation for enstatite from oxides (MgO and SiO2) = ΔGf(enstatite, oxides) is about -35.4 J/mole at room temperature and pressure. Gibbs Energy values are, most often today, given in units of joules/mole or (less commonly) calories/mole.
The ΔGf values given above for enstatite are both negative. This means that enstatite is more stable than, and will form from, the separate elements or separate oxides. Some of the energy produced will be given off as heat; some will contribute to entropy.
Gibbs Free Energies are Relative Values
Gibbs free energies are relative values, not absolute values. They allow us to compare energies of different phases but individual values by themselves have no significance. Because Gibbs Energy values are relative, we can arbitrarily assume some values in order to calculate others. So, by convention the ΔGf for any pure element is assumed to be as zero.
The Gibbs Free Energy of Reaction
We can calculate the Gibbs Free energy of any reaction by summing the energies of the right-hand side of the reaction and subtracting the energies of the left hand side. For example, we can write a reaction describing the formation of enstatite from separate elements:
Mg + Si + 3O = MgSiO3 (rxn 1)
The ΔG for reaction (1) is the Gibbs Free Energy of formation of enstatite from the elements (-1,460.9 J/mole at room temperature and pressure):
ΔG1 = ΔGf(enstatite, elements) = ΔGf(enstatite) - ΔGf(Mg)-ΔGf(Si)-3ΔGf(O) (Eqn 1)
All reactions have an associated ΔGrxn value but the values vary with pressure and temperature (T). If we wish to make thermodynamic calculations involving ΔGf, we must either look the values up in a book for the P-T conditions of interest, or calculate the values using standard equations.
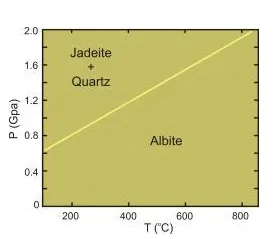
The reaction of Albite to Jadeite + Quartz. At 400° the reaction takes place at about 1.18 GPa.
Determining the Location of Metamorphic Reactions
Consider the reaction: albite = jadeite + quartz (rxn 2)
For this reaction:
ΔG2 = ΔGf (jadeite, elements) + ΔGf (quartz, elements) - ΔGf (albite, elements) (Eqn 2)
or
ΔG2 = ΔGf (jadeite, oxides) + ΔGf (quartz, oxides) - ΔGf (albite, oxides) (Eqn 3)
These two values will always come out to be the same, but most reference books list values relative to the elements. If we look up ΔGf values in a reference book, we will find that:
At 400° C and 1.0 GPa: ΔG2 > 0
At 400° C and 1.4 GPa: ΔG2 < 0
So, at 400° C and 1.0 GPa, albite is the stable form, but at 1.4GPa albite will react to form jadeite + quartz. At about 400°C and 1.18 GPa, on the reaction line, ΔG2 = 0.
For any reaction under a given set of P-T conditions: If the ΔGrxn < 0, the reaction proceeds to the right. If the ΔGrxn >0, the reaction proceeds to the left. The univariant reaction curve that separates the two assemblages actually represents the locus of points where ΔGrxn = 0