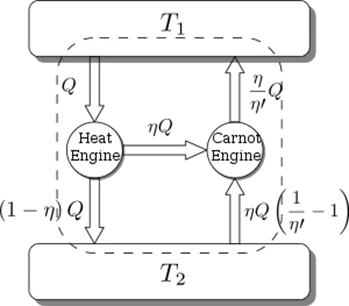
Carnot’s Theorem

This theorem states that no engine working between two given temperatures can be more efficient than a reversible engine working between the same two temperatures and that all the reversible engines working between the same two temperatures have the same efficiency, whatever the working substance may be. According to the Carnot theorem, the reversible engine will always have a greater efficiency than the irreversible one. The reversible heat engine operates on a reverse cycle and functions as a heat pump (or refrigerator).
The Carnot cycle is reversible representing the upper limit on the efficiency of an engine cycle. Practical engine cycles are irreversible and thus have inherently lower efficiency than the Carnot efficiency when operating at the same temperatures.One of the factors determining efficiency is the addition of to the working fluid in the cycle and its removal. The Carnot cycle achieves maximum efficiency because all the heat is added to the working fluid at the maximum temperature.
The Efficiency of Carnot’s Cycle
The Carnot cycle is reversible representing the upper limit on the efficiency of an engine cycle. Practical engine cycles are irreversible and thus have inherently lower efficiency than the Carnot efficiency when operating at the same temperatures.One of the factors determining efficiency is the addition of to the working fluid in the cycle and its removal. The Carnot cycle achieves maximum efficiency because all the heat is added to the working fluid at the maximum temperature.
Carnot cycle

The Carnot engine cycle when acting as a heat engine consists of the following steps:
· Reversible isothermal expansion of the gas at the “hot” temperature.
· Isentropic (reversible adiabatic) expansion of the gas.
· Reversible isothermal compression of the gas at the “cold” temperature.
· Isentropic compression of the gas.