What is Thermodynamic Property – Definition
Within thermodynamics, a physical property is any property that is measurable, and whose value describes a state of a physical system. Our goal here will be to introduce thermodynamic properties, that are used in engineering thermodynamics. These properties will be further applied to energy systems and finally to thermal or nuclear power plants.

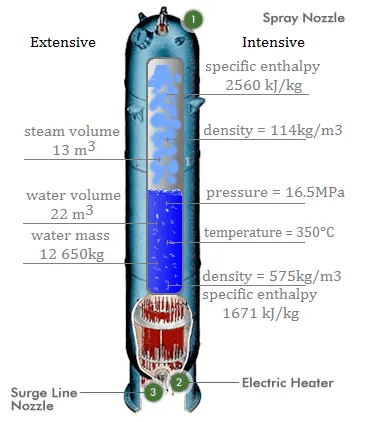
Extensive and intensive properties of medium in the pressurizer.
In general, thermodynamic properties can be divided into two general classes:
· Enthalpy
· Entropy
· Gibbs Free Energy
· Heat Capacity
· Internal Energy
· Mass
· Volume
· Compressibility
· Density
· Specific Enthalpy
· Specific Entropy
· Specific Heat Capacity
· Pressure
· Temperature
· Thermal Conductivity
· Thermal Expansion
· Vapor Quality
· Specific Volume
Specific properties of material are derived from other intensive and extensive properties of that material. For example, the density of water is an intensive property and can be derived from measurements of the mass of a water volume (an extensive property) divided by the volume (another extensive property). Also heat capacity, which is an extensive property of a system can be derived from heat capacity, Cp, and the mass of the system. Dividing these extensive properties gives the specific heat capacity, cp, which is an intensive property.
Specific properties are often used in reference tables as a means of recording material data in a manner that is independent of size or mass. They are very useful for making comparisons about one attribute while cancelling out the effect of variations in another attribute.