
What is Kelvin Planck Statement?
It states that “It is impossible to construct a device which operates on a cycle and produces no other effect than the transfer of heat from a single body in order to produce work.” Which means that It is impossible to construct an engine whose sole purpose is to convert the heat from a high-temperature source/reservoir into an equal amount of work. This is a special case of the second law of thermodynamics.

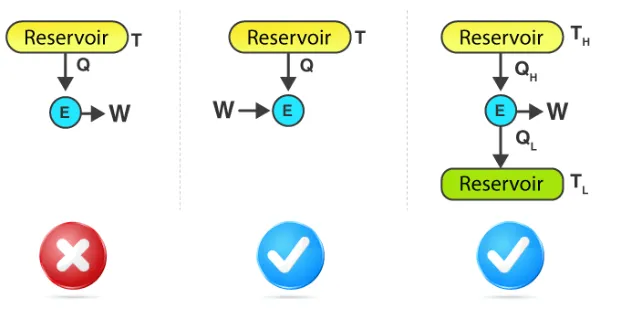
We know that Heat(Q) and Work(W) are the two forms of energy. Both follow the S.I unit Joules and both are interconvertible. Which means, Work can be converted into work and vise-versa. Here, work can be fully converted into heat but heat cannot be fully converted into work. Hence work is called as high-grade energy and heat is called as low-grade energy.
Kelvin-Planck statement is are two different statements given by Lord Kelvin and Planck. They are known as Kelvin statement and Planck statement.
Kelvin statement stated that it is not possible to derive mechanical effect from any matter by cooling it below the highest cooling temperature of the surrounding objects.
Planck statement states that the total sum of entropies for the reversible system remains constant.
Clubbing both these statement Kelvin-Planck statement was derived.
Kelvin-Planck statement is also known as heat engine statement from the second law of thermodynamics states that it is not possible to design a device which works on a cycle and produce no other effect other than heat transfer from a single body for the production of work.
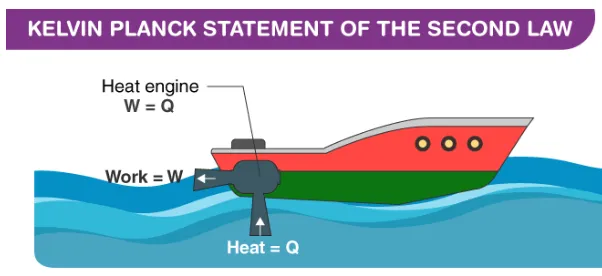
Kelvin-Planck statement example
A hypothetical device called perpetual motion machine of the second kind (PMMSK) was introduced by Wilhelm Ostwald which would perform work exclusively by absorbing energy as heat from a body.
Working of Heat Engine:
For the operation of heat engine, the working fluid has to continuously exchange heat between heat sink/reservoir with low temperature QL and heat source/reservoir with high temperature QH. The prime parameter involved here is efficiency.
The efficiency of a heat engine is “The amount of useful work obtained(output) for a given amount of input”
In general efficiency tells the heat transfer process. It is the ratio of “how much you get out” to “how much you put in”
Efficiency η=−W−QH =QL−QHQL =1−QLQH
In the absence of heat sink, that is: QL=0. Then efficiency
η=1−0QH=1
This implies that efficiency is 100%. Which is not true according to the second law of thermodynamics. Thus, no heat engine is 100% efficient.
η<1 |