First Law of Thermodynamics
The laws of thermodynamics are simple to state. Do you know that the human body obeys the laws of thermodynamics? We start to sweat and feel warm when we’re in a room full of people and the sweating becomes excessive if the room size is small. This happens because your body is trying to cool off hence heat transfers from your body in the form of ‘sweat’. This entails the first law of thermodynamics. Interesting? Let us study more.
First Law of Thermodynamics
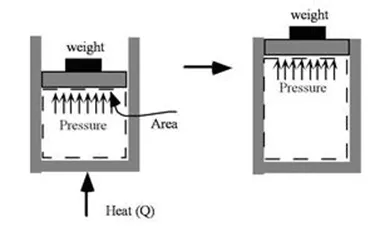
The first law of thermodynamics states that the total energy of an isolated system is constant. Energy can be transformed from one form to another, but can neither be created nor destroyed.

According to this law, some of the heat given to system is used to change the internal energy while the rest in doing work by the system. Mathematically,
ΔQ=ΔU+ΔW
where,
ΔQ = Heat supplied to the system
ΔW= Work done by the system.
ΔU = Change in the internal energy of the system.
If Q is positive, then there is a net heat transfer into the system, if W is positive, then there is work done by the system. So positive Q adds energy to the system and positive W takes energy from the system.
It can also be represented as ΔU=ΔQ−W
We can say that internal energy tends to increase when heat is given to the system and vice versa.
Thermodynamic Cycles
In an isolated system, the total energy remains the same. In a thermodynamic cycle, the net heat supplied to the system equals the net work done by the system. Like for example, the batteries we use convert chemical energy into electrical enrrgy. Also the electric bulbs transform electrical energy into light energy.
Work done on, or by a gas, depends not only on the initial and final states of the gas but also on the process, or the path which produces the final state. Similarly, the amount of heat transferred into, or from a gas also depends on the initial and final states and the process which produces the final state.
The internal energy is just a form of energy like the potential energy of an object at some height above the earth, or the kinetic energy of an object in motion. In the same way that potential energy converts in to kinetic energy while conserving the total energy of the system, the internal energy of a thermodynamic system converts in to either kinetic or potential energy. Like potential energy, the internal energy can be stored in the system. The first law of thermodynamics allows for many possible states of a system to exist, but only certain states are found to exist in nature.
Limitations of First Law of Thermodynamics
· The limitation of the first law of thermodynamics is that it does not say anything about the direction of flow of heat.
· It does not say anything whether the process is a spontaneous process or not.
· The reverse process is not possible. In actual practice, the heat doesn’t convert completely into work.If it would have been possible to convert the whole heat into work, then we could drive ships across the ocean by extracting heat from the water of the ocean.