COMPRESSIBILITY FACTOR
Compressibility factor, usually defined as Z = pV/RT, is unity for an ideal gas. It should not be confused with the isothermal compressibility coefficient. In most engineering work, the compressibility factor is used as a correction factor to ideal behavior. Thus, vreal = Z vid is used to calculate the actual volume, vreal, as the product of the compressibility factor and the ideal gas volume, all at the same pressure and temperature. Z is most commonly found from a generalized compressibility factor chart as a function of the reduced pressure, pr = p/pc, and the reduced temperature, Tτ = T/Tc where pr and Tr are the reduced variables and the subscript 'c' refers to the critical point.
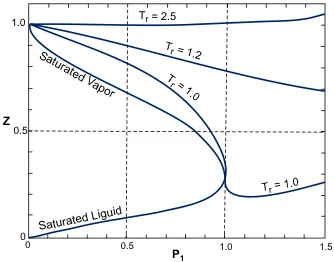
Figure 1 shows the essential features of a generalized compressibility factor chart. The most widely-used compressibility factor charts are apparently those of Nelson and Obert (1954, 1955). These have been extended [see, e.g., Liley (1987)] to include the saturated liquid. A three-parameter correlation Z = f(Pr, Tr, ω), where ω = acentric factor = −log10 pr (Tr = 0.7) −1, involves the use of two compressibility factor charts so that Z = Z0(pr,Tr) + wZl(pr, Tr). [See, e.g., Sonntag, R. E. and van Wylen, G. J. (1991).]
Figure 1. Generalized compressibility factor chart.