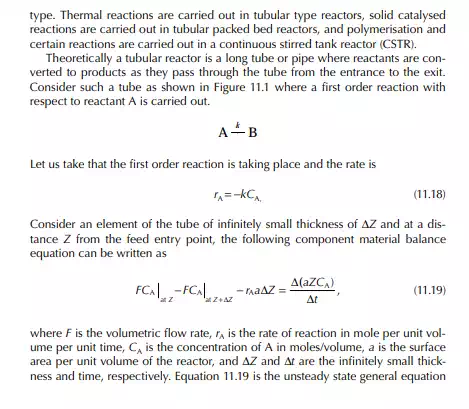
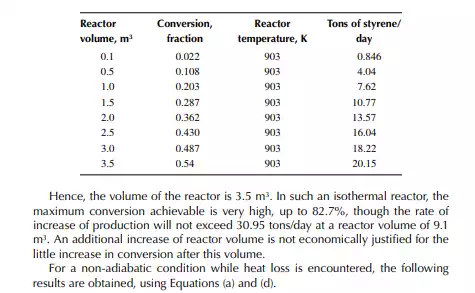
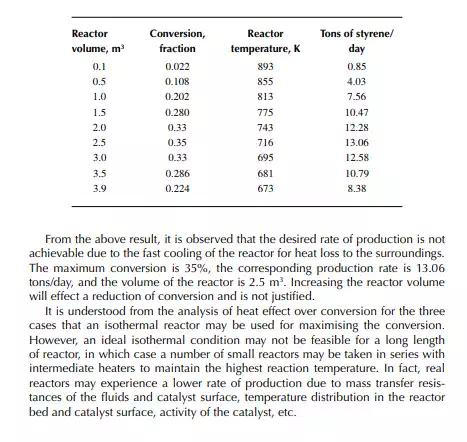
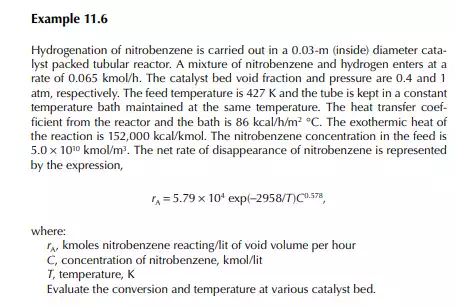
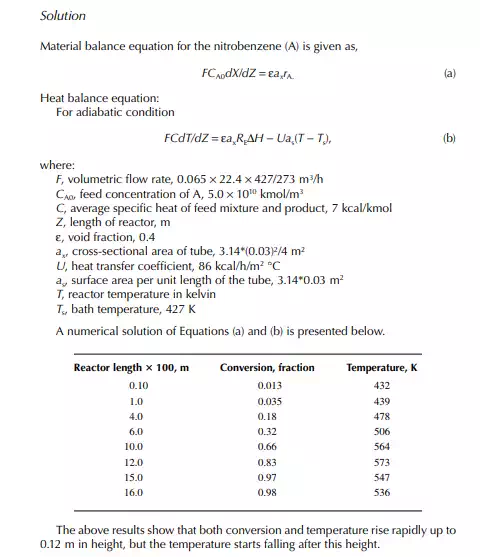
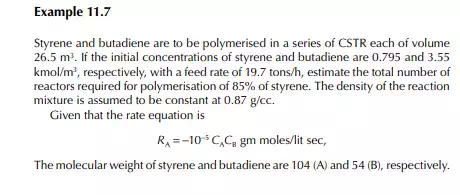
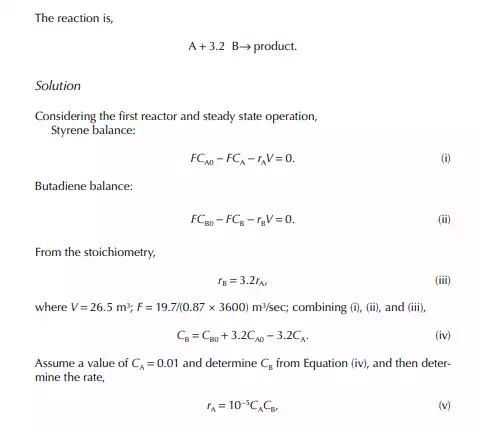
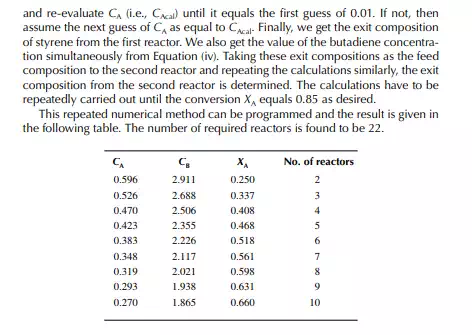
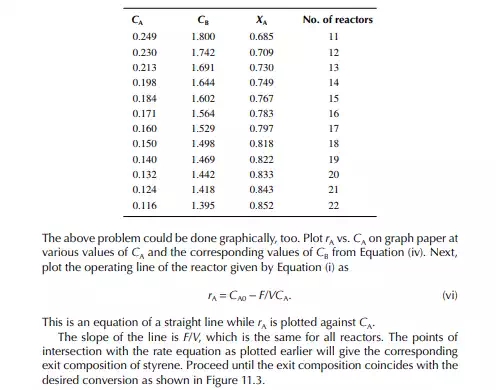
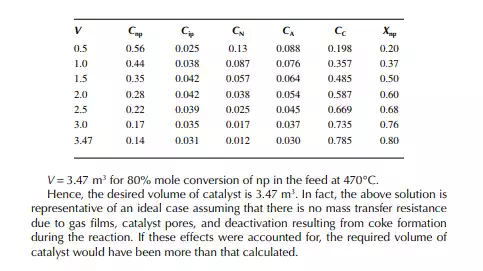
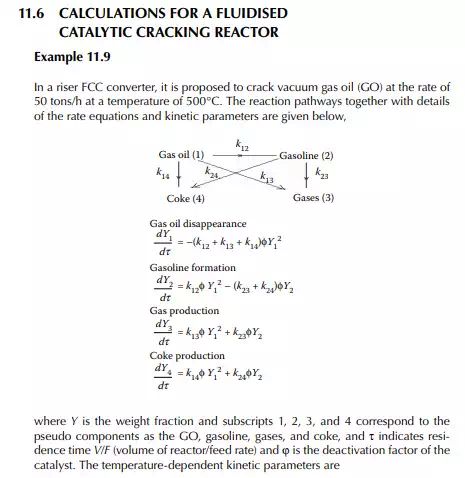
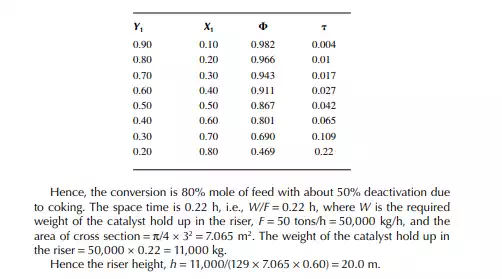
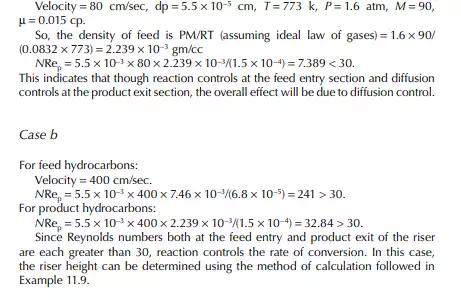
Introduction to Reactor Calculations
11.1 REACTORS IN REFINERIES AND PETROCHEMICAL PLANTS
A reactor is a piece of equipment in which reactions are carried out to manufacture product materials chemically and physically different from the raw materials (reactants) put into it. These are vessels made of specially selected materials to withstand the operating temperature, pressure, and the chemical corrosion resulting from the reacting fl uids present within the reactors. Common reactions encountered in petroleum refi ning are thermal and catalytic cracking, hydrogenation, desulfurisation, isomerisation, dehydrogenation, etc., which are fast reactions as compared to polymerisation reactions involved in resin or plastic manufacturing plants. The reactors may be either batch or continuous types depending on the reaction type. Usually, slow reactions are carried out in batch reactors whereas fast reactions are carried out in continuous fl ow reactors. Catalytic reactors using a solid catalyst are predominantly packed bed fl ow reactors. Fluidised bed reactors use a circulating catalyst with a steam or inert gas fl uidising medium coupled with simultaneous regeneration facilities. Reactors with a circulating catalyst without fl uidisation are also in use. A circulating catalyst through a liquid medium falls in the category of slurry reactors.
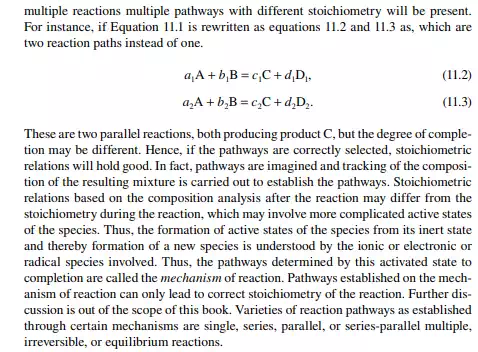
11.2 REACTION STOICHIOMETRY, MECHANISM, AND PATHWAYS
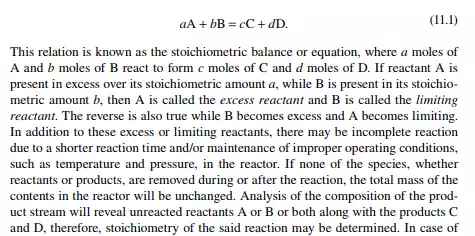
If two chemical species, A and B, react to form a product, C and D, which have different chemical and physical properties than those of the reactants A and B, the total mass of the system will remain unchanged according to the Law of Conservation of Matter. This can be represented by a simple mathematical relation as
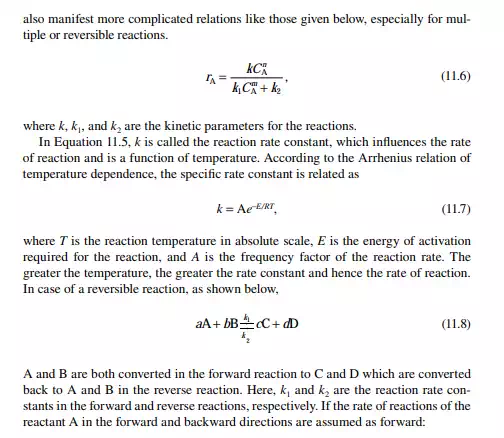

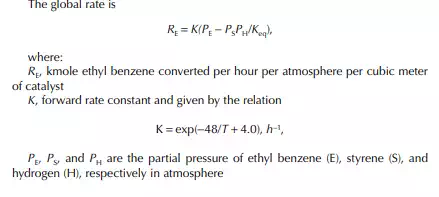
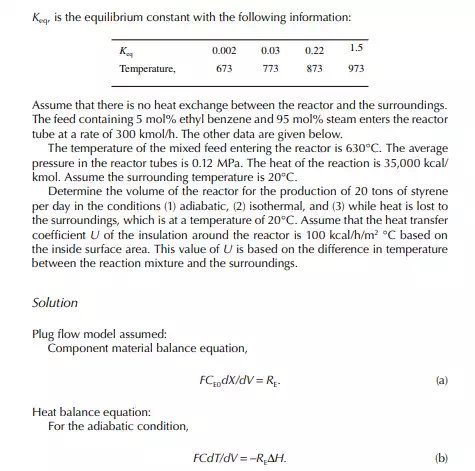
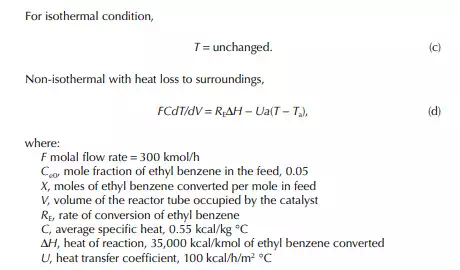
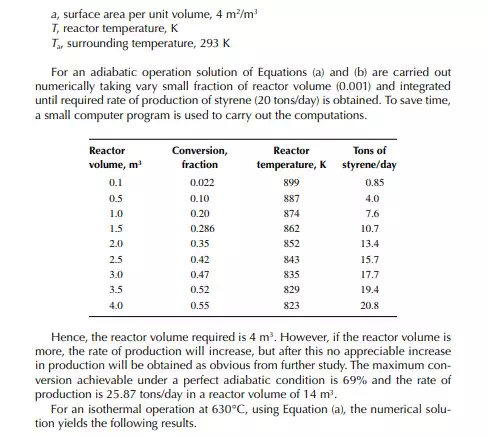
The ratio, k1/k2 = K, is known as the equilibrium constant. If the value of K is greater than unity, it indicates that the forward reaction is favoured more than the reverse reaction. The equilibrium rate constant is a strong function of the temperature, like the specifi c rate constants. An increase in reaction rate will enhance forward and backward reactions equally. Catalytic activity will also enhance the reactions equally in both directions. The compositions in Equation 11.13 are the equilibrium compositions and will vary with operating temperature. The rate of reaction is also a function of operating pressure, while any gaseous component is present as the reactant.
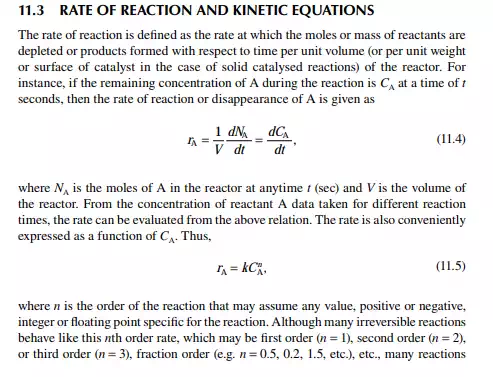
11.4 BATCH, CONTINUOUS STIRRED TANK REACTOR, AND PLUG FLOW REACTOR CONCEPTS
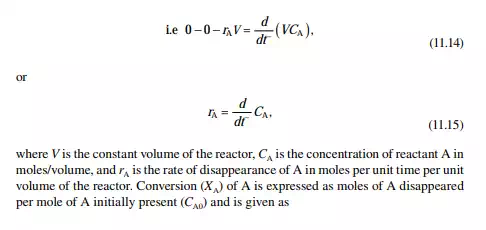
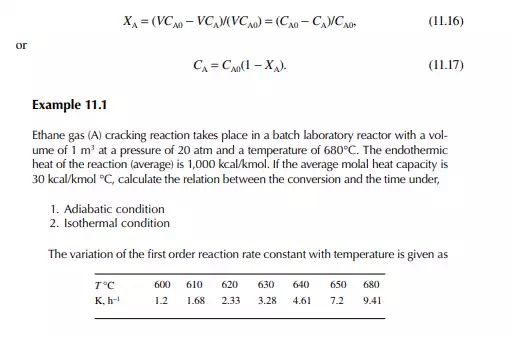
A batch reactor is a reaction vessel where reactants, liquid or gas or both (with or without a catalyst as the case may be), are fi lled in the vessel initially and closed. Then, proper operating conditions, e.g., temperature and pressure, are raised to carry out a reaction and left for a certain period for the reaction to continue. Thus, the reaction is a time-varying process in such a reactor. The reaction time may vary from reaction to reaction. The reactor is then cooled to release the products. The amount of product obtained is called the batch quantity. The reactor is then cleaned and refi lled with the reactants and the reaction is repeated. This type of operation involves much manual activity and a large amount of time is lost to fi lling and discharging, raising the operating temperature and pressure, and fi nally cooling for withdrawal of the products. A good amount of heat, especially for a high temperature reaction, is lost every time during cooling before product withdrawal. A large production rate batch reaction process is not economical where a fl ow or continuous reactor must be used. However, high value products in small quantities are manufactured in batch reactors. Theoretically, the rate equation and composition of reactants and products can be related in a constant volume batch reactor as Input – output – disappearance of reactant “A” = rate of depletion of reactant within the reactor
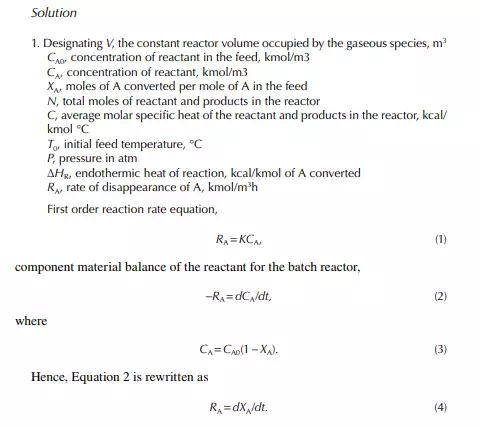

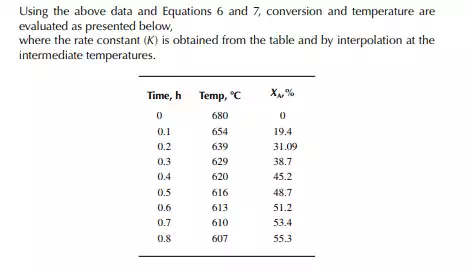
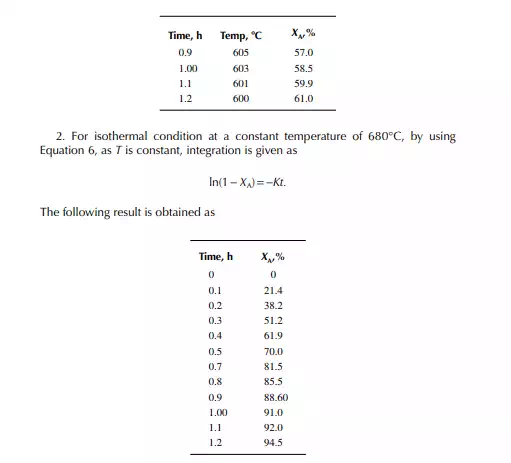
This indicates that the maintenance of a constant highest possible reaction temperature increases the conversion manifold as compared to an adiabatic reactor. This helps in reducing the required volume of the reactor as compared to an adiabatic one. A fl ow reactor is a reactor where reactants and products enter and leave simultaneously and continuously. Steady state operating conditions, such as temperature, pressure, feed fl ow, and composition, are required to be maintained to have uniform product quality. In this fl ow process, the operating conditions have to be raised at the start to the desired level before the reactants enter. After this is complete, the reactants are introduced. At the beginning, the product quality varies with time to yield a uniform composition. This period required for raising steady state operating conditions and uniform product quality is known as the start-up period. Production is then continued for a long time and is stopped only when repair or maintenance is required or when the supply of reactants or demand for products falls short. Usually, a continuous run period varies from 6 months to 1 year while maintenance or repair is made for the entire plant. There are continuous operations without any shut down where maintenance is done online, e.g., fl uidised catalytic cracking (FCC), circulating catalytic reformer, and catalytic reformer with a swing reactor. Reactors are either tubular or stirred tank