Introduction to Extraction
10.1 EXTRACTION PRINCIPLE
If two liquids, a solvent and a solute, are in a homogeneous solution and a third liquid is introduced into the solution, there will be two liquid layers separated into two homogeneous solutions as shown in Figure 10.1. This type of phenomenon will occur only for certain selected third liquids, which solubilise any one or both of the components, which were formerly in the homogeneous solution. Phase separation occurs due to the difference in density of the resulting solutions. This phenomenon is utilised for separating two miscible liquid components from a solution and such a method is called extraction. If the solute (A), the carrier liquid (B) in the feed mixture, and the pure solvent (C) are brought in intimate contact by thorough mixing, the solute will transfer to the solvent due to the concentration gradient set up between the feed and the solvent. This transfer (mass transfer) process will continue until the equilibrium concentration of the solute between the feed and the solvent is achieved, as long as the feed and the solvent are in intimate contact. Extraction is applicable for separating components that are diffi cult to separate by distillation, settling, or other means. For instance, if the boiling points of the components are very close or the desired components are too sensitive to high temperature,
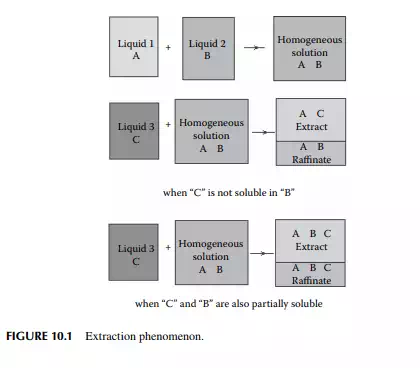
extraction should be used as the best method for separation. When all the components involved in extraction are in liquid phases, the process is known as liquid extraction. While the feed is a solid mixture containing the solute, extraction by a liquid solvent is carried out and the process is known as leaching. For example, separation of aromatic hydrocarbons from vacuum distillates by furfural (solvent) is an example of liquid extraction, whereas removal of paraffi nic hydrocarbons from waxy distillates by ketone (solvent) is an example of leaching. In refi neries and petrochemical plants, extraction processes also involve more than one solute and more than one solvent.
10.2 EXTRACTION PROCESS
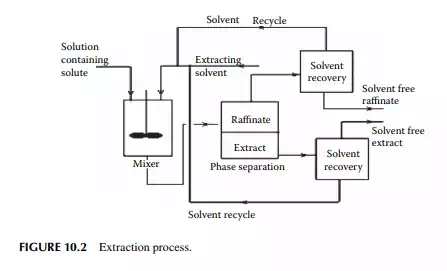
Any extraction process requires three operating steps, i.e., mixing, separating, and solvent recovery. The solvent and the feed must be mixed intimately to transfer the solute to the solvent. After mixing, the fi nal mixture is allowed to settle, while two phases containing the solvent rich phase or the extract and the solvent lean phase or raffi nate are separated. These phases will separate by gravity or by centrifuge settler. Usually, the solvent is also partially soluble in the feed and vice versa and as a result both the extract and raffi nate phases will contain solvent, solute, and the carrier components. The next step will be to recover the solvent from these phases usually by distillation. Finally, the extract phase will yield solute and the raffi nate phase will be the feed liquids nearly free from solutes. The success of any extraction process depends on various factors as listed below. The steps for an extraction process are schematically explained in Figure 10.2.
10.3 DEFINITION OF TERMS RELATED TO EXTRACTION
10.3.1 PARTITION COEFFICIENT
If the amount of solute in the extract is x and that in raffi nate is y, then the ratio y/x is known as the partition or distribution coeffi cient or equilibrium constant. The greater this value, the greater the separation of the solute from the original mixture.
10.3.2 PARTIAL SOLUBILITY
If the solvent (C) solubilises the solute components (A) alone without interfering the carrier components (B), then the original mixture will be separated from the extract containing no solvent. But many of the solvents also solubilise the carrier (B) components, causing ternary mixture of A, B, and C in both the extract and raffi nate phases.
10.3.3 SOLVENT TO FEED RATIO
If the amount of solvent and feed is Wc and Wf , then the ratio Wc/Wf is the solvent to feed ratio. If the solubility of the desired solute in the solvent is high, a lower solvent to feed ratio will be required. If the solvent is also partially soluble in the carrier liquid, a higher solvent to feed ratio will be desirable.
10.3.4 SOLVENT RECOVERY
Solvents must be recovered from the extract and the raffi nate as: 1. Solvents are not desirable in the fi nal products 2. Solvents are costly 3. Solvents cannot be disposed of as they will pollute the environment A solvent is commonly recovered by distillation or stripping. Care must be taken that the quality of the solvent should not degrade during this recovery process nor should it contain a high concentration of residual solute, which will otherwise make it unfi t for reuse.
10.3.5 SEPARATION OF PHASES
Usually, the greater the density difference between the extract and raffi nate phases, the quicker the separation by gravity and the cheaper it will be. For small density difference, a costlier separation, e.g., centrifuging, is essential to speed up the process. The viscosity of the phases also plays an important role during separation. A high viscosity of one or both of the phases reduces the speed of separation. Other factors, such as selectivity, solvent power, and critical solution temperature (CST), must also be taken into consideration for the selection of the solvent.
10.3.6 SELECTIVITY
Selectivity is the ratio of the distribution coeffi cient of the solute to that of the solute free carrier. For instance, if paraffi n and aromatic hydrocarbons are present in feed, then the selectivity (β) of the solvent will be defi ned by the ratio of distribution coeffi cients of aromatic hydrocarbon to paraffi n hydrocarbon.
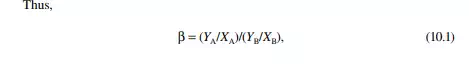
where YA and YB are the mass or mole fraction of the solute and solute carrier, respectively, in the extract phase, and XA and XB are the mass or mole fractions of the solute and carrier, respectively, in the raffi nate phase.
10.3.7 SOLVENT POWER
Solvent power is defi ned as the ratio of mass of solvent free extract to the mass of solvent used. Thus, the greater the dissolving power of the solvent, the greater this ratio will be. However, high solvent power also dissolves undesired feed components and thus reduces the yield of raffi nate. High solvent power also makes less impure extract. Therefore, a compromise must be made between the solvent power and selectivity while the solvent is selected.
10.3.8 CRITICAL SOLUTION TEMPERATURE
When a solvent is mixed with the feed hydrocarbons, phase separation occurs in a certain range of temperatures. As the temperature is raised, the separated phases start remixing and yield a homogeneous solution at a certain temperature, known as the critical solution temperature (CST). Hence, a solvent of high CST must be selected so that phase separation will occur in a wide range of temperatures. The CST for the same solvent is also affected by the nature of the components involved, e.g., straight chain or paraffi ns have a higher CST as compared to branched chain or ring compounds and aromatics. Thus, the CST increases as the feed (containing paraffi ns and aromatics) is gradually freed from aromatics. Hence, the temperature gradient in an extractor plays an important role in extraction.
10.4 PHASE EQUILIBRIUM IN THE EXTRACTION PROCESS
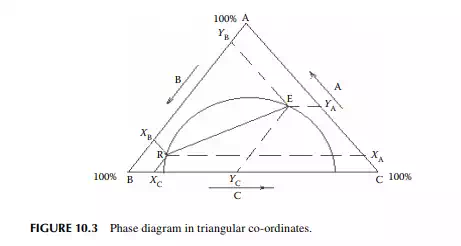
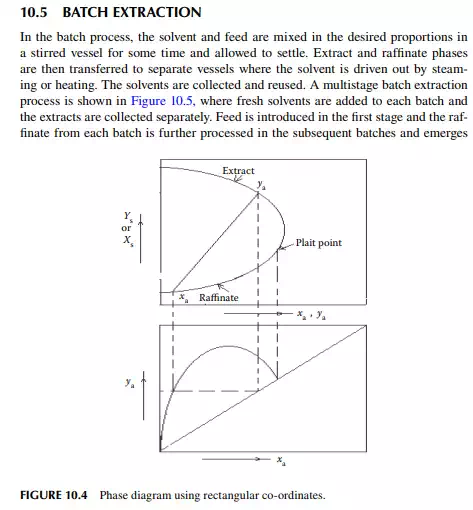
For a mutually soluble solvent in a binary feed of solute and carrier, the resulting raffi nate and extract phases each become a ternary mixture of solute, solvent, and the carrier feed solvent. Theoretically, if infi nite time of separation of the phases is allowed, the phases will attain equilibrium. Thus, the composition of each component in the raffi nate and extract will be related by their corresponding distribution constants. A ternary mixture is best represented graphically by a triangular diagram as shown in Figure 10.3, where points E and R are the locations of the equilibrium compositions of the components A, B, and C, as Y and X, respectively. The line ER in the diagram connects the equilibrium compositions in the extract and raffi nate phases. Alternatively, phase relationships are also conveniently handled in rectangular diagrams as shown in Figure 10.4, where the solvent concentrations (extract and raffi nate phases, ordinates) per unit of solvent free mixture are plotted against the solvent free solute compositions in the raffi nate and extract phases along the x-axis. The equilibrium points, ya and xa, are located on this phase diagram with the help of the lower plot, where ya and xa are the solvent free solute compositions in the extract and raffi nate phases, respectively. At the plait point, ya and xa are equal and hence no separation is possible when this point is reached.
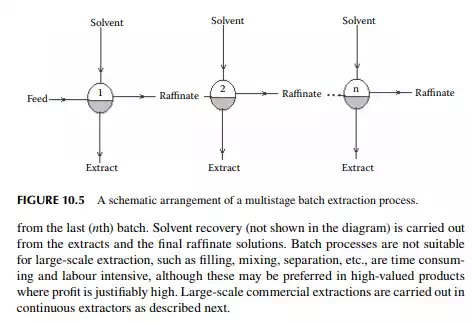
10.6 CONTINUOUS EXTRACTION
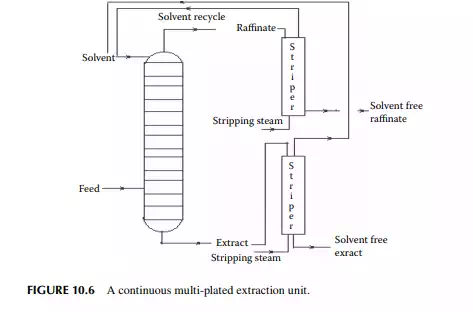
Continuous extraction is carried out in a tall plated tower where the feed and solvent are treated countercurrently. Simultaneous mixing and continuous phase separation occur while one phase is drawn from the top and the other from the bottom of the tower. Each plate provides intimate contact between the phases and enhances the enrichment of the extract and raffi nate phases leaving the plates. A countercurrent plated tower extractor is shown in Figure 10.6, where the feed is introduced near the bottom of the tower and the solvent from the top. Usually, the solvent has a higher density than the feed and hence the extract becomes heavier than the raffi nate. Therefore, the extract is drawn from the bottom and the raffi nate from the top of the tower. The temperature gradient along the tower plays an important role for both in the extraction and phase separation. Each plate provides intimate mixing between the extract falling from the top plate with the raffi nate rising from the plate below it. Theoretically, the compositions of the extract and the raffi nate leaving a plate are in equilibrium. However, in practice, equilibrium compositions are not achieved because of inadequate mixing and the time of separation. This is compensated by using a higher number of plates than the ideal number of plates to achieve maximum extraction.
10.6.1 COMPUTATION OF NUMBER OF PLATES
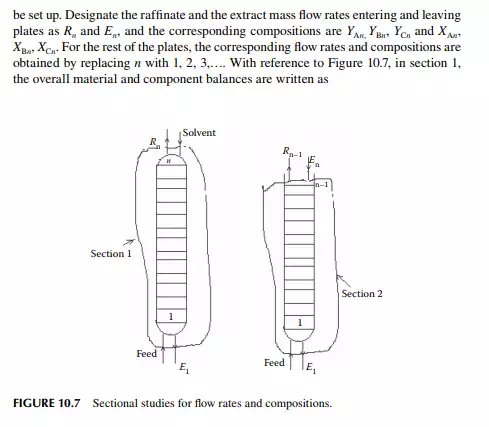
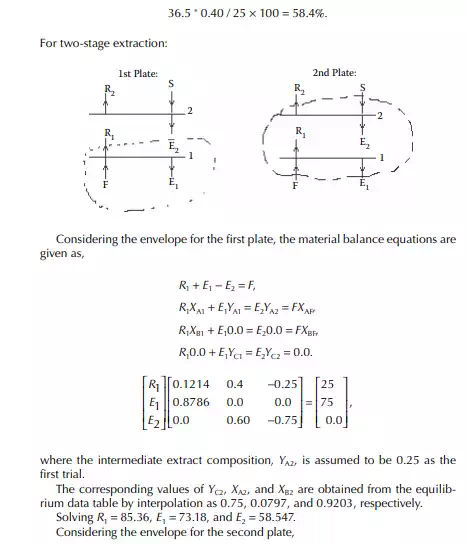
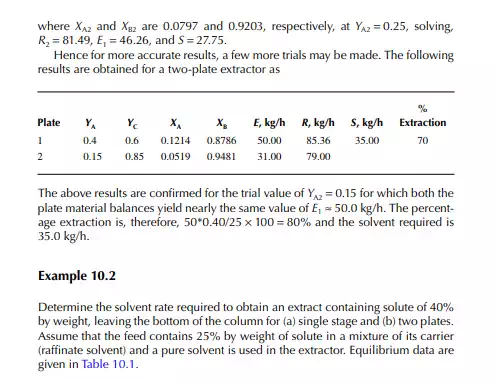
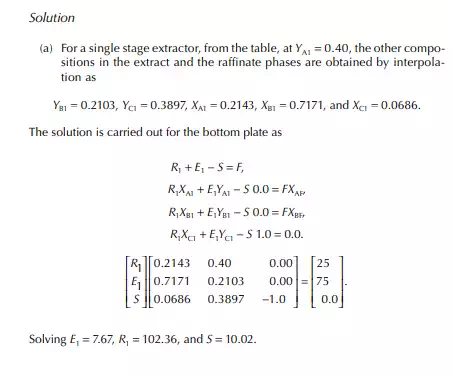
Consider an extraction process involving a feed containing solute A in a carrier solvent (raffi nate solvent) B and an extracting solvent C which are countercurrently treated in a multiplated column. If the number of plates in a column is n as measured from the bottom as the fi rst plate, the feed entry section, the following equations can
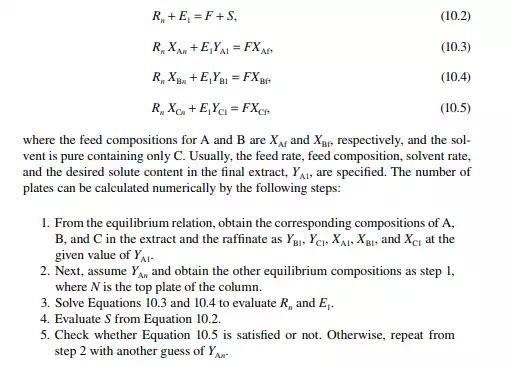
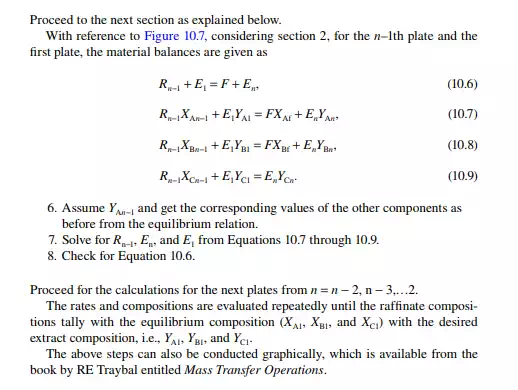
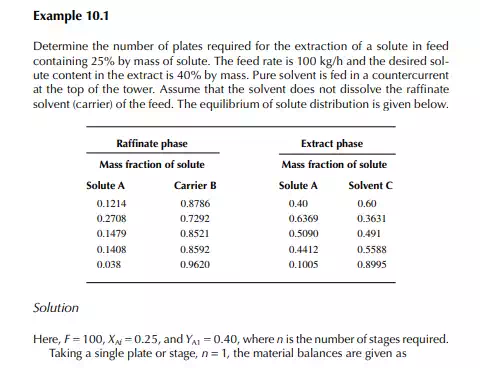
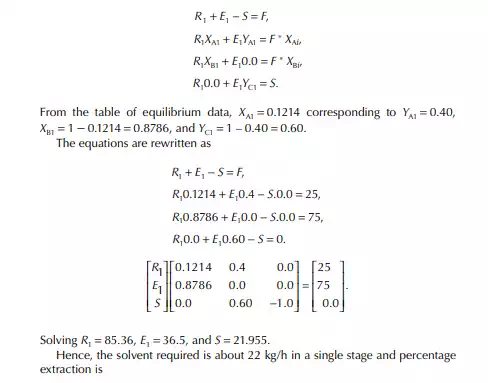
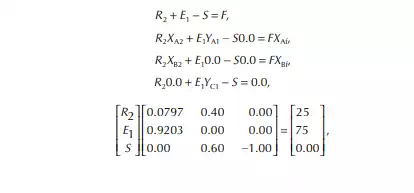

QUESTIONS
1. What are the basic differences between extraction and distillation?
2. Defi ne the terms solute, solvent, extract, and raffi nate.
3. Differentiate the extraction processes between the operations in a plated tower and the operations in a series of mixer/separator tanks