Fluid catalytic cracking: some background
A short history
Commercial production of petroleum dates back to 1859, when Colonel Edwin L. Drake found “rock oil” in Titusville (PA, USA). The initial petroleum products were refined in very simple refineries without conversion capability. At the beginning of the 20th century, the number of cars propelled by an internal combustion engine sharply increased, and a shortage of gasoline developed.
Thermal cracking, in which the unused fractions in the higher boiling range were converted to gasoline-range molecules, was first introduced in 1913, by Burton at Standard Oil of Indiana. However, the gasoline produced by this process was of relatively poor quality. Additives like tetra-ethyl lead, discovered in the 1920's by Midgley, could improve the “octane number” of gasoline, but other solutions were required. The first technical embodiment of catalytic cracking was introduced in 1915, when McAfee at Gulf Refining Company developed a catalyst based on aluminum chloride.6 However, this process was not economically feasible, and was abandoned.
In the 1920's, French engineer Houdry experimented with the conversion of lignite to useful products, and found that clay minerals could convert his lignite-based oil to a fuel similar to gasoline. This was the advent of catalytic cracking as we know it today. Houdry moved to the USA and developed his process with the Socony-Vacuum Oil company (which later became Mobil Oil Company), and eventually the first catalytic cracker operating the Houdry process, which processed 15 000 barrels of petroleum per day, was started up in 1936 in Paulsboro (NJ, USA). The first full-scale commercial plant went on-stream in 1937 at Sun Oil's refinery in Marcus Hook (PA, USA).8 The catalyst was replaced by a synthetic silica-alumina already in the early 1940's, and the process, which produced very high quality fuels, was very quickly developed to produce aviation fuel for the allied war effort in the Second World War. The original Houdry process made use of a fixed bed reactor.
In 1938, a consortium called Catalytic Research Associates (originally Standard Oil of New Jersey, Standard Oil of Indiana, M. W. Kellogg Co., and I. G. Farben) set out to develop a new cracking process At the beginning of the 2nd World War, I. G. Farben was dropped from the Consortium, and Anglo-Iranian Oil Co. Ltd, Royal Dutch-Shell Co., The Texas Co. and Universal Oil Products Co. (UOP) joined. A pilot plant based on a powdered catalyst moving through a pipe coil reactor and a regenerator was built in Baton Rouge (LA, USA). The 100 barrels per day unit was called PECLA-1 (Powdered Experimental Catalyst, Louisiana). In about a year, the system was developed to commercial stage, and mid-1942, the first commercial FCC unit (PCLA-1) was started up. This system was based on an up-flow reactor and regenerator and used a clay-based catalyst. It was based on work of Lewis and Gilliland, working with Standard Oil Company of New Jersey, who suggested that a low velocity gas flow through a powder might “lift” it enough to cause it to flow in a manner similar to a liquid.
The system was extremely successful, and with ongoing developments at the end of the war, 34 FCC units were in operation in the USA. PCLA No. 3, which was the second unit at Baton Rouge, was started up in June, 1943. This unit is still in operation today, and is the oldest operating FCC unit, as the PCLA-1 unit was shut down in 1963.
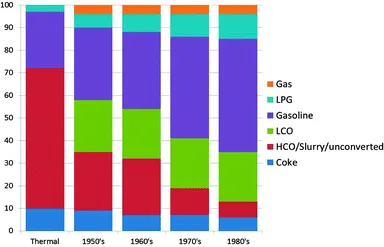
As mentioned before the initial FCC process used clay-based catalysts. Improvements were soon made, and synthetic amorphous SiO2–Al2O3 or SiO2–MgO-based catalysts were developed already in the 1940's. The reason for this was an improved selectivity to the desired products, as can be observed in using data from . The graph shows a combined effect of activity increase and selectivity improvement.
Fig. 2 The effect of improving reactor and catalyst technology on the product selectivity for the FCC process for four decades in the 1900's. Color-coding: gas: orange; LPG: light blue; gasoline: purple; LCO: green; HCO/slurry/unconverted: red; and coke: dark blue. Data are obtained from ref. 15 and 16.
In the early 1960's and 1970's, synthetic crystalline microporous aluminosilicates (i.e. zeolites) were invented at the laboratories of Union Carbide and Mobil Oil Corporation. The first of these relevant to FCC was synthetic faujasite (IUPAC structure code FAU ), or zeolite Y (Linde Y), invented by Breck at Union Carbide. Zeolite Y in various improved forms has been the main cracking component of FCC catalysts since 1964. The initial embodiment was Mg-stabilized, while the currently used rare earth (RE)-stabilized zeolite Y was introduced fairly quickly after that. A second zeolite that has found large-scale application in FCC is zeolite ZSM-5 (IUPAC structure code MF), which was invented in 1973 by Argauer and Landolt at Mobil Oil Corporation.22 The main application of zeolite ZSM-5 has been in FCC operation targeting an increased propylene yield. clearly shows that the introduction of zeolite materials in FCC catalyst formulations resulted in a drastic increase in the gasoline yield in the 1970s and 1980s. The books by Venuto and Habib23 and Scherzer give good accounts of the history and backgrounds of the FCC process up to the 1980's.