Classification of fuels
A fuel is a substance which gives heat energy on combustion. A fuel contains carbon and hydrogen as main combustible elements. fuel is any material that can be made to react with other substances so that it releases chemical or nuclear energy as heat or to be used for work. heat energy released by reactions of fuels is converted into mechanical energy via a heat engine. Other times the heat itself is valued for warmth, cooking, or industrial processes, as well as the illumination that comes with combustion. Fuels are also used in the cells of organisms in a process known as cellular respiration, where organic molecules are oxidized to release usable energy.
Types of Fuels
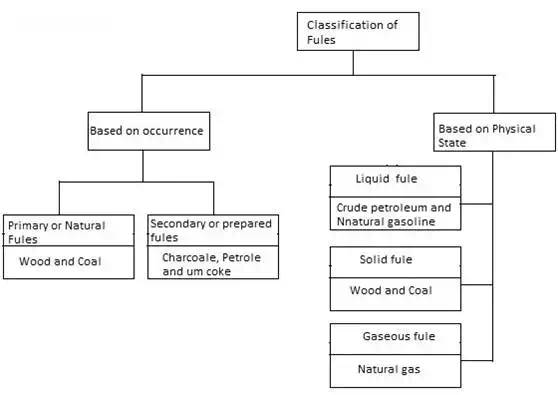
The based on physical states, fuel can be classified into three types.
Liqued Fuels
Liquid fuels like furnace oil and are predominantly used in industrial applications. Most liquid fuels in widespread use are derived from the fossilized remains of dead plants and animals by exposure to heat and pressure in the Earth's crust. However, there are several types, such as hydrogen fuel (for automotive uses), ethanol, jet fuel and biodiesel which are all categorized as a liquid fuel.
Types of liquid fuel
· Petroleum
· Oils from distillation of petroleum
· Coal tar
· Shale-oil
·
Alcohols, etc.
The properties of liquid fules
|
DENSITY |
Density is defined as the ratio of the mass of the fuel to the volume of the fuel at a reference temperature of 15°C. The unit of measurement for density is kg/m3 and measured by a hydrometer. It is important for assessing ignition qualities and other quantitative calculations. |
|
SPECIFIC GRAVITY |
The specific gravity is a ratio, which is defined as the ratio of the weight of a given volume of oil to the weight of the same volume of water at a given temperature. The density of fuel, relative to water is called specific gravity. E.g. Light diesel oil has specific gravity as 0.85 - 0.87, furnace oil has 0.89 - 0.95. |
|
VISCOSITY |
The viscosity of a fluid is a measure of its internal resistance to flow. Viscosity depends on the temperature and decreases as the temperature increases. Every oil has its own temperature - viscosity relationship and measurement by viscometer. It is important characteristic for storage and use of fuel oil. It influences the degree of pre-heating required for handling, storage and satisfactory atomization. Highly viscous oils may become difficult to pump, hard to light the burner, and difficult to handle. the low atomization may result in the formation of carbon deposits on the burner tips/walls. The pre-heating is necessary for proper atomization. |
|
FLASH POINT |
The flash point of a fuel is the lowest temperature at which the fuel can be heated so that the vapour gives off flashes momentarily when an open flame is passed over it. The 66 °C is the flash point for furnace oil. |
|
POUR POINT |
It is the fuel's lowest temperature at which it will pour or flow when cooled under prescribed conditions. It is a rough estimation of the lowest temperature at which fuel oil is ready to be pumped. |
|
SPECIFIC HEAT |
Specific heat is the amount of calories needed to raise the temperature of 1 kg of oil by 10C. The unit of specific heat is kcal/kg0C. It varies from 0.22 to 0.28 depending on the oil specific gravity. |
|
CALORIFIC VALUE |
The calorific value measures the heat or energy produced. Gross calorific value (GCV) assumes all vapour produced during the combustion process is fully condensed and Net calorific value (NCV) assumes the water leaves with the combustion products without fully being condensed. Fuels should be compared based on the net calorific value. The calorific value of fuel oils is much more consistent compare to coal (solid fule), for example kerosene and diesel oil got the GCV 11,100 and 10,800 kCal/kg respectively. |
|
SULPHUR |
The amount of sulphur in the fuel oil depends on the source of the crude oil and on the refining process. The sulphur content for the residual fuel oil is in the order of 2 - 4 %. |
|
ASH CONTENT |
The ash value is related to the inorganic material or salts(compounds of sodium, vanadium, calcium, magnesium, silicon, iron, aluminium, nickel etc.) in the fuel oil and ash levels in distillate fuels are negligible. the residual fuels have higher ash levels. The ash has an erosive effect on the burner tips, causes damage to the refractories at high temperatures and gives rise to high temperature corrosion and fouling of equipments. |
|
CARBON RESIDUE |
Carbon residue indicates the tendency of oil to deposit a carbonaceous solid residue on a hot surface like burner and injection nozzle when its vaporizable constituents evaporate. The residual oil contains carbon residue of 1% or higher. |
|
WATER CONTENT |
The water content are low when it is supplied because the product at refinery site is handled hot. the water content can be maximum 1% which the upper limit.the water content can cause damage to the inside surfaces of the furnace during combustion especially if it contains dissolved salts or it can cause spluttering of the flame at the burner tip, possibly extinguishing the flame, reducing the flame temperature or lengthening the flame. |

Gross calorific values for different fuel oils
|
Fuel oils |
Gross Calorific Value (kCal/kg) |
|
Fuel Oil |
11,100 |
|
Diesel Oil |
10,800 |
|
L.D.O |
10,700 |
|
Furnace Oil |
10,500 |
|
LSHS |
10,600 |
Solid Fuels
Solid fuel refers to various types of solid material that are used as fuel to produce energy and provide heating, usually released through combustion. Coal is classified into three major types; anthracite, bituminous, and lignite. However, there is no clear demarcation between them. Coal is further classified as semi-anthracite, semi-bituminous, and sub-bituminous. Anthracite is the oldest coal from a geological perspective. It is a hard coal composed mainly of carbon with little volatile content and practically no moisture.
Types of solid fuel
· Wood
· Coal
· Oil shale
· Tanbark
· Bagasse
· Straw
· Charcoal
· Coke
·
Briquettes
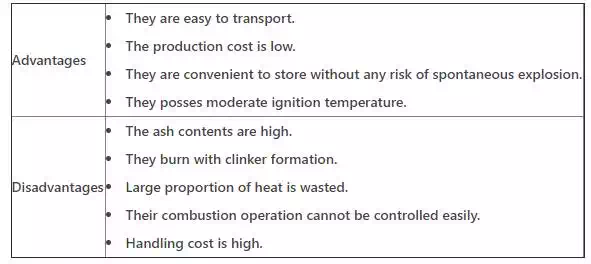
Solid fuels has the following advantages and Disadvantages:
Woods Characteristics
The woods are very easily available and most commonly used
solid fuel. The woods are used as fuel from ancient time after the discovery of
the fire. The 39 Types of fuels and their Characteristics wood is used in
almost every village, town and cities in India. The wood is used for industrial
purposes. Constituents of Wood is vegetable tissue of trees and bushes. The
wood consists of mainly cellular tissue & lignin. it also consists of
lesser parts of fat & tar and sugar.
Calorific Value of wood
Engineer A. Marjhevskee determined the calorific values of different kinds of wood with the help of the samples taken out from the same tree at different distances from centre as follows.
|
Kinds of Wood |
Lowest Calorific Value (cal/kg) |
Highest Calorific Value (cal/kg) |
|
Oak |
4729 |
4750 |
|
Birch |
4695 |
4831 |
|
Elm |
4674 |
4833 |
|
Alder |
4745 |
4839 |
|
Pine |
4818 |
5310 |
|
Fir |
4887 |
4900 |
|
Lrch |
4775 |
4840 |
Coal classification
Coal is classified into three types as follows, even there is
no clear demarcation between them:
1. Anthracite
2. Bituminous
3. Lignite.
The Coal is further classified as
semi-anthracite, semi-bituminous and sub-bituminous. the anthracite is the
oldest coal from a geological perspective. It is a hard coal composed mainly of
carbon with little volatile content and without moisture. The lignite is the
youngest coal from a geological perspective and it is a soft coal composed
mainly of volatile matter(combustible constituents of coal that vaporize when
coal is heated). and moisture content with low fixed carbon(carbon in its free
state, not combined with other elements).
The coals used in Indian industry are bituminous
and sub-bituminous coal. The chemical composition of coal has a strong
influence on its combustibility.
Chemical and physical properties of coal
The chemical properties of coal refer to the various
elemental chemical constituents such as carbon, hydrogen, oxygen, and sulphur.
The physical properties of coal include the
heating value, moisture content, volatile matter and ash.
Gaseous Fuel
Fuel gas is any one of a number of fuels that under ordinary conditions are gaseous. Many fuel gases are composed of hydrocarbons ,hydrogen, carbon monoxide, or mixtures thereof. Such gases are sources of potential heat energy or light energy that can be readily transmitted and distributed through pipes from the point of origin directly to the place of consumption. Fuel gas is contrasted with liquid fuels and from solid fuels, though some fuel gases are liquefied for storage or transport. While their gaseous nature can be advantageous, avoiding the difficulty of transporting solid fuel and the dangers of spillage inherent in liquid fuels, it can also be dangerous.
Types of gaseous fuel
· Natural gas
· Liquefied Petroleum gas (LPG)
· Refinery gases
· Methane from coal mines
· Fuel gases made from solid fuel
· Gases derived from coal
· Gases derived from waste and biomass
· Blast furnace gas
· Gases made from petroleum
· gases from oil gasification
·
Gases from some fermentation process
Gaseous fuels has the following advantages and Disadvantages over solid or liquid fuels :

Properties of gaseous fuels
The fuel should be compared based on their net calorific value and especially true for natural gas because increased hydrogen content results in high water formation during combustion.
1. LPG
LPG may be defined as those hydrocarbons, which are gaseous
at normal atmospheric pressure but may be condensed to the liquid state at
normal temperature by the application of moderate pressures. The LPG is a
predominant mixture of propane and butane with a small percentage of
unsaturated, some lighter C2 and heavier C5 fractions. The propane (C3H8),
Propylene (C3H6), iso-butane (C4H10) and Butylene (C4H8) are included in the
range of LPG.The liquid LPG evaporates to produce about 250 times volume of
gas.
LPG vapour is denser than air for example butane
is about two times heavier then air and propane is about 1.5 times heavier then
air. Consequently the vapours may flow along the ground and into drains sinking
to the lowest level of the surroundings and be ignited at a considerable
distance from the source of leakage. There should be adequate ground level of
ventilation where LPG is stored therefore LPG cylinders should not be stored in
cellars or basements which have no ventilation at ground level.
2. Natural gas
Natural gas
has high calorific value and requiring no storage facilities. It mixes with air
readily and does not produce smoke or soot. It did not contains sulphur. It is
lighter than air and disperses into air easily in case of leak.
The methane is the main constituent of natural
gas and it is about 95% of the total volume. The other components are Ethane,
Propane, Butane, Pentane, Nitrogen, Carbon Dioxide, and traces of other gases.
In these gases a very small amounts of sulphur compounds are also present. The
properties of methane are used when comparing the properties of natural gas to
other fuels because methane is the largest component in natural gas.