Acid–base catalysis
Acid–base catalysis, acceleration of a chemical reaction by the addition of an acid or a base, the acid or base itself not being consumed in the reaction. The catalytic reaction may be acid-specific (acid catalysis), as in the case of decomposition of the sugar sucrose into glucose and fructose in sulfuric acid; or base-specific (base catalysis), as in the addition of hydrogen cyanide to aldehydes and ketones in the presence of sodium hydroxide. Many reactions are catalyzed by both acids and bases.
The mechanism of acid- and base-catalyzed reactions is explained in terms of the Brønsted–Lowryconcept of acids and bases as one in which there is an initial transfer of protons from an acidic catalyst to the reactant or from the reactant to a basic catalyst. In terms of the Lewis theory of acids and bases, the reaction entails sharing of an electron pair donated by a base catalyst or accepted by an acid catalyst. Acid catalysis is employed in a large number of industrial reactions, among them the conversion of petroleum hydrocarbons to gasoline and related products. Such reactions include decomposition of high-molecular-weight hydrocarbons (cracking) using alumina–silica catalysts (Brønsted–Lowry acids), polymerization of unsaturated hydrocarbons using sulfuric acid or hydrogen fluoride (Brønsted–Lowry acids), and isomerization of aliphatic hydrocarbons using aluminum chloride (a Lewis acid).
Among industrial applications of base-catalyzed reactions is the reaction of diisocyanates with polyfunctional alcohols in the presence of amines, used in the manufacture of polyurethane foams.
Acid–base catalysis
Acids (including Lewis acids) and bases act as powerful catalysts for a great variety of chemical reactions, in the laboratory, in industry, and in processes occurring in nature. Historically, catalytic action was regarded as one of the essential characteristics of acids, and the parallel occurrence of catalytic action and electrical conductivity was one of the compelling pieces of evidence in establishing the theory of electrolytic dissociation as the basis of acid–base behaviour at the end of the 19th century.
Acid–base catalysis was originally thought of in terms of a mysterious influence of the acid or base, but it is now generally believed to involve an actual acid–base reaction between the catalyst and the reacting substance, termed the substrate, with the catalyst being regenerated at a later stage of the reaction. Moreover, knowledge of reaction mechanisms is now sufficient to suggest detailed sequences of reactions for many acid- or base-catalysis reactions, most of these sequences being at least plausible and in many instances well established.
In most acid–base reactions the addition or removal of a proton does not bring about any drastic change in the structure of the molecule or in its stability or reactivity. It is a characteristic of reactions catalyzed by acids or bases, however, that the addition or removal of a proton either makes the substrate unstable, so that it decomposes or rearranges, or that it causes the substrate to become reactive toward some other species present in the system. In cases of rearrangement, the regeneration of the catalyst often involves the removal or addition of a proton at a site other than that at which the initial addition or removal took place. It is not necessary that the substrate in an acid- or base-catalyzed reaction should itself have marked acid–base properties, since even a very small extent of initial acid–base reaction may be enough to bring about the subsequent change.
Instances of acid–base catalysis are numerous indeed; a few examples are given here, as follows:
Isomerization of olefins, acid-catalyzed
Unsaturated compounds frequently rearrange reversibly under the influence of acids to give products in which the double bond occurs in a new location. The interconversion of 2-butene and 1-butene is shown here:
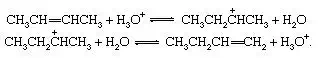
Reversible dehydration of alcohols, acid-catalyzed. Under the influence of acids, alcohols generally undergo loss of water to give olefinic products. The dehydration of ethanol to ethylene occurs as follows:
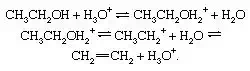
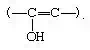
Keto–enol tautomerism, acid- and base-catalyzed
Acids and bases both bring about the establishment of an equilibriumbetween ketones (or aldehydes) and their enol forms, which contain a hydroxyl group directly attached to a doubly bonded carbon atom:
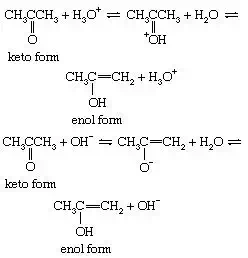
The interconversion between the two forms is called keto–enol tautomerism. The reaction cannot always be observed directly, since the enol form may not reach measurable concentrations, even at equilibrium, but the highly active enol may be detected by its reaction with various reagents, notably the halogens (bromine, for example). Keto–enol tautomerization of acetone can be brought about by acid or base catalysis, as follows:
Aldol
condensation, base-catalyzed
Self-condensation of aldehydes, the so-called aldol condensation, occurs readily, when catalyzed by bases, to give β-hydroxy aldehydes. The prototype of this reaction is the conversion of acetaldehyde to β-hydroxybutyraldehyde, or aldol. The first step of this reaction is the production of an enolate ion (as in formation of the keto–enol tautomeric mixture), but this anion then reacts with a second molecule of acetaldehyde to give the product as shown below:
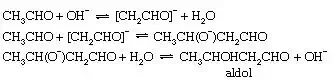
These examples illustrate the importance of acid–base catalysis in organic reactions. The equations have been written in terms of H3O+ and OH− as the acid and base catalysts, respectively, and these are certainly the most important catalysts in aqueous solution. For many of these reactions (especially isomerization of olefins and dehydration of alcohols), there is ample evidence that other acids or bases also can act as catalysts. This behaviour is known as general acid–base catalysis. It appears particularly clearly in inert solvents such as benzene, in which catalysis by molecular acids and bases is frequently observed despite the absence of detectable quantities of ions derived from the solvent. Acidic groups, such as sulfonic acid (−SO3H) and carboxylic acid groups (−CO2H), attached to a solid molecular framework (as in some ion exchange resins) also act as heterogeneous catalysts for many chemical reactions.
The above examples show that proton-transfer processes can play a specific part in reaction mechanisms and, in these and similar instances, it is doubtful whether any uncatalyzed or spontaneous reaction of the same type can take place. Apparent evidence to the contrary can usually be explained by catalysis by solvent molecules or by adventitious acidic or basic impurities.
Lewis acids can exert a catalytic effect in two different ways. In the first of these they interact with hydrogen-containing compounds present in the system to assist the release of a proton to the substrate. For example, the polymerizationof olefins by Lewis acids, such as boron trifluoride (BF3), aluminum chloride(AlCl3), and titanium tetrachloride (TiCl4), is believed to be caused by their interaction with proton acids (for example, traces of water) and the olefin to give a carbonium ion, which then reacts further with more olefin:
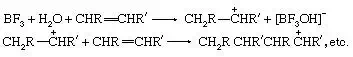
In the second mode of action, the Lewis acid acts directly on the substrate, and by withdrawing electrons converts it into a reactive form. A typical example is the action of catalysts like aluminum chloride on alkyl halides to produce carbonium ions: RCl + AlCl3 → R+ + [AlCl4]−. The carbonium ion can then react further with other substances, for example, aromatic hydrocarbons. The same type of catalysis probably occurs with many solid oxide catalysts (for example, aluminosilicates), although it is often difficult to decide whether the catalytic action of these materials is due to centres with a deficiency of electrons or to acidic hydroxyl groups.