Catalytic Promoters
Substances which themselves are not catalysts, but when mixed in small quantities with the catalysts increase their efficiency are called as promoters or activators.
(i) For example, in Haberís process for the synthesis of ammonia, traces of molybdenum increases the activity of finely divided iron which acts as a catalyst.
(ii)
In the manufacture of methyl alcohol from water gas 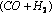 , chromic oxide
, chromic oxide 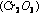 is used as a promoter with the catalyst zinc oxide
is used as a promoter with the catalyst zinc oxide 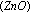 .
.
Catalytic poisons : Substances which destroy the activity of the catalyst by their presence are known as catalytic poisons.
(i)
For example, the presence of traces of arsenious oxide 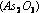 in the reacting gases reduces the activity of platinized
asbestos which is used as catalyst in contact process for the manufacture of
sulphuric acid.
in the reacting gases reduces the activity of platinized
asbestos which is used as catalyst in contact process for the manufacture of
sulphuric acid.
(ii)
The activity of iron catalyst is destroyed by the presence
of 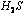 or
or 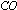 in the synthesis of ammonia by Haberís process.
in the synthesis of ammonia by Haberís process.
(iii)
The platinum catalyst used in the oxidation of hydrogen is
poisoned by 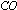 .
.
Change of temperature alters the rate of catalytic reaction as it does for the same reaction in absence of catalyst : By increasing the temperature, there is an increase in the catalytic power of a catalyst but after a certain temperature its power begins to decrease. A catalyst has thus, a particular temperature at which its catalytic activity is maximum. This temperature is termed asoptimum temperature.
A positive catalyst lowers the activation energy
(i) According to the collision theory, a reaction occurs on account of effective collisions between the reacting molecules.
(ii) For effective collision, it is necessary that the molecules must possess a minimum amount of energy known as activation energy (Ea).
(iii) After the collision molecules form an activated complex which dissociate to yield the product molecules.
(iv) The catalyst provides a new pathway involving lower amount of activation energy. Thus,
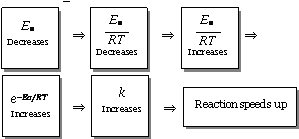
larger number of effective collisions occur in the presence of a catalyst in comparison to effective collisions at the same temperature in absence of a catalyst. Hence the presence of a catalyst makes the reaction to go faster.
(v)
Figure shows that activation energy  , in absence of a catalyst is higher than the activation energy
Ea, in presence of a catalyst.
, in absence of a catalyst is higher than the activation energy
Ea, in presence of a catalyst.
(vi)
 and
and 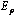 represent the average energies of reactants and products.
The difference gives the value of
represent the average energies of reactants and products.
The difference gives the value of 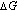 , i.e.,
, i.e., 
