Adsorption
Define Adsorption
Adsorption is defined as the deposition of molecular species onto the surface. The molecular species that gets adsorbed on the surface is known as adsorbate and the surface on which adsorption occurs is known as adsorbent. Common examples of adsorbents are clay, silica gel, colloids, metals etc.
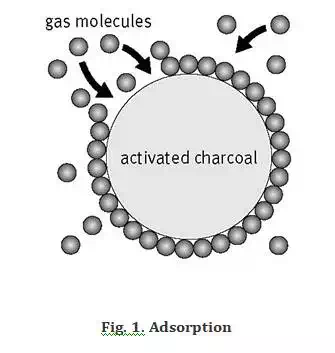
Adsorption is a surface phenomenon. The process of removal of adsorbent from the surface of adsorbate is known as desorption.
Difference between Absorption and Adsorption
|
Absorption |
Adsorption |
|
Substance penetrates the surface |
Surface phenomenon |
|
It occurs at uniform rate |
Rate increases initially than it decreases |
|
It is unaffected by temperature |
It is affected by temperature |
|
It is an endothermic process |
It is an exothermic process |
|
It is same throughout the material |
Concentration on the surface of adsorbent is different from that in the bulk |
Mechanism of Adsorption
The amount of heat evolved when one mole of the adsorbate is adsorbed on adsorbent is called enthalpy of adsorption. Adsorption is an exothermic process and enthalpy change is always negative. When adsorbate molecules are adsorbed on the surface, freedom of movement of molecules become restricted and this results in decrease in entropy. Adsorption is a spontaneous process at constant pressure and temperature, thus Gibb’s free energy is also decreased.
Types of Adsorption
There are two types of Adsorption – Physical Adsorption or Physiosorption and Chemical Adsorption or Chemisorption.
Physical Adsorption
It involves adsorption of gases on solid surface via weak van der Waal’s forces.
Characteristics of Physical Adsorption
- There is no specificity in case of physical adsorption. Every gas is adsorbed on the surface of the solid.
- Nature of the adsorbate. Easily liquefiable gases are strongly adsorbed physically.
- Physical adsorption is reversible in nature. If pressure is increased volume of gas decreases as a result more gas is adsorbed. So, by decreasing the pressure, gas can be removed from the solid surface. Low temperature promotes physical adsorption and high temperature decreases the rate of adsorption.
- More surface area more is the rate of adsorption. Porous substances and finely divided metals are good adsorbents.
- Physical adsorption is an exothermic process.
- No activation energy is needed.
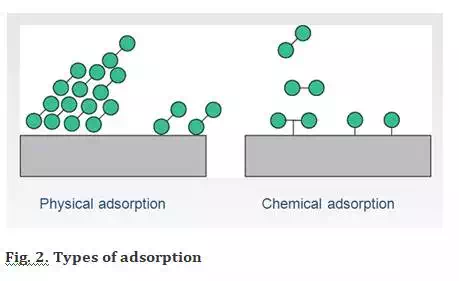
Chemical Adsorption or Chemisorption
When the gas molecules or atoms are held to the solid surface via chemical bonds, this type of adsorption is chemical adsorption or chemisorption.
Characteristics of Chemical Adsorption
- This type of adsorption is specific as compared to physical adsorption. Adsorption occurs only if there is formation of chemical bonds between the adsorbate and adsorbent.
- Chemical adsorption is irreversible. It is an exothermic process but the process occurs slowly at low temperature. Chemisorption is accompanied by increase in temperature. High pressure promotes chemisorption.
- Chemisorption increases with increase in surface area.
- Due to chemical bond formation enthalpy of chemisorption is high.
- Activation energy is needed.
- It results in unimolecular layer.
Adsorption isotherms
Adsorption isotherm is a graph or a relation between the amounts of adsorbate adsorbed on the surface of adsorbent and pressure at a constant temperature.
Different adsorption isotherm was studied by different scientists-
Freundlich Adsorption Isotherm
Freundlich proposed an empirical relationship between amount of gas adsorbed by unit mass of adsorbent and pressure at a particular temperature. Following equation was proposed for freundlich adsorption isotherm-
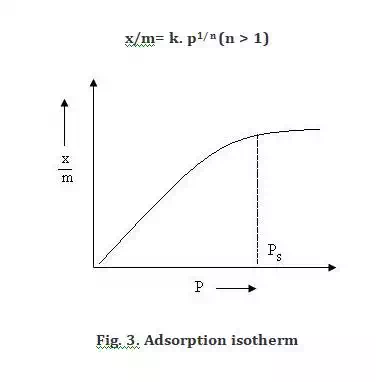
x is the mass of the gas adsorbed
m is the mass of the adsorbent
p is the pressure
k and n are constants which depends on the nature of the adsorbent and the gas at a particular temperature.
Taking log of the above equation, the following equation will be observed
log x/m = log k +1/n log p
x/m is plotted on y axis and log p is on x axis. If straight line is observed than only freundlich isotherm is verified.
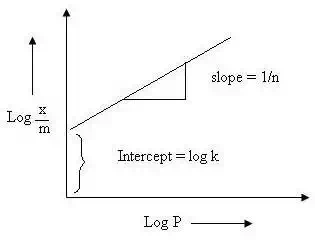
Fig. 4. Freundlich isotherm
Slope gives 1/n and intercept gives log k. The value of 1/n varies from 0 to 1.
If 1/n is 0, adsorption is independent of pressure.
If 1/n is 1, adsorption changes with pressure.
Adsorption from solution phase
Solids also adsorb from solutions. For example, when a solution of acetic acid in water is mixed with charcoal, some of the acid is adsorbed by the charcoal.
Characteristics of adsorption from the solution phase-
- Adsorption decreases with increase in temperature.
- More surface area more is the rate of adsorption.
- Adsorption also depends on concentration of solute in a solution.
- Adsorption also depends on nature of adsorbate and nature of adsorbent.
Freundlich explains the adsorption from solution phase using concentration of the solution instead of pressure
x/m = kC1/n
Taking log of the above equation, the following reaction will be obtained-
log x/m = log k +1/n log C
x/m against log C will give straight line.
Factors affecting the Adsorption
- Temperature is an important factor that affects the adsorption. Adsorption occurs best at low temperature. As adsorption is an exothermic process, low temperature will derive the forward reaction.
- Adsorption increases with increase in pressure up to certain extent until saturation is reached. After saturation, has achieved no more adsorption will occur irrespective of the pressure applied. The relationship between the extent of adsorption and temperature at any constant pressure is called Adsorption Isobar.
- As adsorption is a surface phenomenon, surface area will increase the rate of adsorption.
- Easily liquefiable gases are easily adsorbed.
Applications of Adsorption
- High vacuum can be created using adsorption strategy. For creating vacuum activated charcoal is used.
- Gas masks used in coal mines are based on adsorption principle. These gas masks are used to adsorb poisonous gases. This makes the air purified for breathing.
- Silica and aluminum gels are used to adsorb moisture to reduce humidity.
- Noble gases can be separated using charcoal as an adsorbent.
- Adsorption of drugs are used to kill germs.
- Chromatographic analysis is based on phenomenon of adsorption.
- Sugar is decolorized by treating sugar solution with charcoal powder. The latter adsorbs the undesirable colors present.
- Adsorption also plays an important role in paint industry. The paint should not contain dissolved gases, as otherwise the paint does not adhere well to the surface to be painted and thus will have a poor covering power.
- This method is also used in the formation of the stable emulsions in cosmetics and syrups.
- The cleaning action of soaps and detergents are also due to adsorption.