Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with thermodynamics, which deals with the direction in which a process occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction.
History
In 1864, Peter Waage and Cato Guldberg pioneered the development of chemical kinetics by formulating the law of mass action, which states that the speed of a chemical reaction is proportional to the quantity of the reacting substances. Van 't Hoff studied chemical dynamics and in 1884 published his famous "Études de dynamique chimique". In 1901 he was awarded by the first Nobel Prize in Chemistry "in recognition of the extraordinary services he has rendered by the discovery of the laws of chemical dynamics and osmotic pressure in solutions". After van 't Hoff, chemical kinetics deals with the experimental determination of reaction rates from which rate laws and rate constants are derived. Relatively simple rate laws exist for zero order reactions (for which reaction rates are independent of concentration), first order reactions, and second order reactions, and can be derived for others. Elementary reactions follow the law of mass action, but the rate law of stepwise reactions has to be derived by combining the rate laws of the various elementary steps, and can become rather complex. In consecutive reactions, the rate-determining step often determines the kinetics. In consecutive first order reactions, a steady state approximation can simplify the rate law. The activation energy for a reaction is experimentally determined through the Arrhenius equation and the Eyring equation. The main factors that influence the reaction rate include: the physical state of the reactants, the concentrations of the reactants, the temperature at which the reaction occurs, and whether or not any catalysts are present in the reaction.
Gorban and Yablonsky have suggested that the history of chemical dynamics can be divided into three eras.[6] The first is the van 't Hoff wave searching for the general laws of chemical reactions and relating kinetics to thermodynamics. The second may be called the Semenov--Hinshelwood wave with emphasis on reaction mechanisms, especially for chain reactions. The third is associated with Aris and the detailed mathematical description of chemical reaction networks.
Factors affecting reaction rate
The reaction rate varies depending upon what substances are reacting. Acid/base reactions, the formation of salts, and ion exchange are usually fast reactions. When covalent bond formation takes place between the molecules and when large molecules are formed, the reactions tend to be slower.
The nature and strength of bonds in reactant molecules greatly influence the rate of their transformation into products.
The physical state (solid, liquid, or gas) of a reactant is also an important factor of the rate of change. When reactants are in the same phase, as in aqueous solution, thermal motion brings them into contact. However, when they are in separate phases, the reaction is limited to the interface between the reactants. Reaction can occur only at their area of contact; in the case of a liquid and a gas, at the surface of the liquid. Vigorous shaking and stirring may be needed to bring the reaction to completion. This means that the more finely divided a solid or liquid reactant the greater its surface area per unit volume and the more contact it with the other reactant, thus the faster the reaction. To make an analogy, for example, when one starts a fire, one uses wood chips and small branches — one does not start with large logs right away. In organic chemistry, on water reactions are the exception to the rule that homogeneous reactions take place faster than heterogeneous reactions ( are those reactions in which solute and solvent not mix properly)
In a solid, only those particles that are at the surface can be involved in a reaction. Crushing a solid into smaller parts means that more particles are present at the surface, and the frequency of collisions between these and reactant particles increases, and so reaction occurs more rapidly. For example, Sherbet (powder) is a mixture of very fine powder of malic acid (a weak organic acid) and sodium hydrogen carbonate. On contact with the saliva in the mouth, these chemicals quickly dissolve and react, releasing carbon dioxide and providing for the fizzy sensation. Also, fireworks manufacturers modify the surface area of solid reactants to control the rate at which the fuels in fireworks are oxidised, using this to create diverse effects. For example, finely divided aluminium confined in a shell explodes violently. If larger pieces of aluminium are used, the reaction is slower and sparks are seen as pieces of burning metal are ejected.
The reactions are due to collisions of reactant species. The frequency with which the molecules or ions collide depends upon their concentrations. The more crowded the molecules are, the more likely they are to collide and react with one another. Thus, an increase in the concentrations of the reactants will usually result in the corresponding increase in the reaction rate, while a decrease in the concentrations will usually have a reverse effect. For example, combustion will occur more rapidly in pure oxygen than in air (21% oxygen).
The rate equation shows the detailed dependence of the reaction rate on the concentrations of reactants and other species present. The mathematical forms depend on the reaction mechanism. The actual rate equation for a given reaction is determined experimentally and provides information about the reaction mechanism. The mathematical expression of the rate equation is often given by
Temperature usually has a major effect on the rate of a chemical reaction. Molecules at a higher temperature have more thermal energy. Although collision frequency is greater at higher temperatures, this alone contributes only a very small proportion to the increase in rate of reaction. Much more important is the fact that the proportion of reactant molecules with sufficient energy to react (energy greater than activation energy: E > Ea) is significantly higher and is explained in detail by the Maxwell–Boltzmann distribution of molecular energies.
The effect of temperature on the reaction rate constant usually obeys the Arrhenius equation ![]() , where A is the pre-exponential factor or A-factor, Ea is the activation energy, R is the molar gas constant and T is the absolute temperature.
, where A is the pre-exponential factor or A-factor, Ea is the activation energy, R is the molar gas constant and T is the absolute temperature.
At a given temperature, the chemical rate of a reaction depends on the value of the A-factor, the magnitude of the activation energy, and the concentrations of the reactants. Usually, rapid reactions require relatively small activation energies.
The 'rule of thumb' that the rate of chemical reactions doubles for every 10 °C temperature rise is a common misconception. This may have been generalized from the special case of biological systems, where the α (temperature coefficient) is often between 1.5 and 2.5.
The kinetics of rapid reactions can be studied with the temperature jump method. This involves using a sharp rise in temperature and observing the relaxation time of the return to equilibrium. A particularly useful form of temperature jump apparatus is a shock tube, which can rapidly increase a gas's temperature by more than 1000 degrees.
Main article: Catalysis
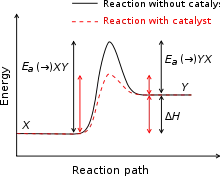
Generic potential energy diagram showing the effect of a catalyst in a hypothetical endothermic chemical reaction. The presence of the catalyst opens a new reaction pathway (shown in red) with a lower activation energy. The final result and the overall thermodynamics are the same.
A catalyst is a substance that alters the rate of a chemical reaction but it remains chemically unchanged afterwards. The catalyst increases the rate of the reaction by providing a new reaction mechanism to occur with in a lower activation energy. In autocatalysis a reaction product is itself a catalyst for that reaction leading to positive feedback. Proteins that act as catalysts in biochemical reactions are called enzymes. Michaelis–Menten kinetics describe the rate of enzyme mediated reactions. A catalyst does not affect the position of the equilibrium, as the catalyst speeds up the backward and forward reactions equally.
In certain organic molecules, specific substituents can have an influence on reaction rate in neighbouring group participation.[citation needed]
Increasing the pressure in a gaseous reaction will increase the number of collisions between reactants, increasing the rate of reaction. This is because the activity of a gas is directly proportional to the partial pressure of the gas. This is similar to the effect of increasing the concentration of a solution.
In addition to this straightforward mass-action effect, the rate coefficients themselves can change due to pressure. The rate coefficients and products of many high-temperature gas-phase reactions change if an inert gas is added to the mixture; variations on this effect are called fall-off and chemical activation. These phenomena are due to exothermic or endothermic reactions occurring faster than heat transfer, causing the reacting molecules to have non-thermal energy distributions (non-Boltzmann distribution). Increasing the pressure increases the heat transfer rate between the reacting molecules and the rest of the system, reducing this effect.
Condensed-phase rate coefficients can also be affected by pressure, although rather high pressures are required for a measurable effect because ions and molecules are not very compressible. This effect is often studied using diamond anvils.
A reaction's kinetics can also be studied with a pressure jump approach. This involves making fast changes in pressure and observing the relaxation time of the return to equilibrium.