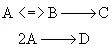Parallel Reactions

The net rate of disappearance of A
Instantaneous selectivity
If α > β use high concentration of A. Use PFR.
If α < β use low concentration of A. Use CSTR.
Series Reactions (p. 283)
Example: Series Reaction in a batch reactor
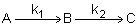
This series reaction could also be written as
Reaction (1)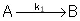 : -r1A=k1CA
: -r1A=k1CA
Reaction (2)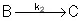 : -r2B=k2CB
: -r2B=k2CB
Mole Balance on every species
Species A: Combined mole balance and rate law for a control volume batch reactor. |
|
Net Rate of Reaction of A | |
| rA=r1A+0 |
Rate Law | |
| r1A=-k1ACA |
Relative Rates | |
| r1B=-r1A |
|
|
Integrating with CA=CA0 at t=0 and then rearranging |
|
Mole Balance | |
Species B: |
|
Net Rate of Reaction of B | |
|
|
Rate Law | |
| r2B=-k2CB |
Relative Rates | |
|
|
|
|
Combine | |
| |
Using the integrating factor, i.f.: |
|
Evaluate | |
|
|
at t = 0, CB = 0 |
|
When should you stop the reaction to obtain the maximum amount of B? Let's see. | |
Optimization of the Desired Product B | |
|
|
Then |
|
Species C | CC = CA0 - CB - CA |
And |
|
Concentration-Time Trajectories
Schemes for maximizing the selectivity for Van Der Vusse Kinetics