
Classification based on the nature of rearrangements of atoms
So far you studied about a chemical reaction and how it can be described as a chemical equation. A large number of chemical reactions are taking place around us every day.Are they taking place in a similar way? No. Each reaction involves different kinds of atoms and hence the way they react also differs. Thus, based on the manner by which the atoms of the reactants are rearranged, chemical reactions are classified as follows.
(a) Combination reactions
A combination reaction is a reaction in which two or more reactants combine to form a compound. It is otherwise called 'synthesis reaction' or 'composition reaction'. When a reactant ‘A’ combines with ‘B’, it forms the product ‘AB’. The generalised scheme of a combination reaction is given below:

Example: Hydrogen gas combines with chlorine gas to form hydrogen chloride gas.
H2(g) + Cl2(g) → 2HCl(g)
Depending on the chemical nature of the reactants, there are three classes of combination reactions:
Element + Element → Compound
In this type of combination reaction, two elements react with one other to form a compound. The reaction may take place between a metal and a non-metal or two non-metals.
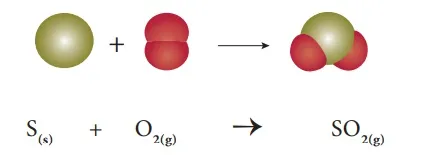
Example 1: When solid sulphur reacts with oxygen, it produces sulphur dioxide. Here both the reactants are non-metals.
Example 2: Sodium, a silvery-white metal, combines with chlorine, a pale yellow green gas, to form sodium chloride, an edible compound. Here one of the reactants is a metal (sodium) and the other (chlorine) is a non-metal.
2Na(s) + Cl2(g) → 2NaCl(s)
Test Yourself:
Identify the possible combination reactions between the metals and non-metals given in the following table and write their balanced chemical equations:
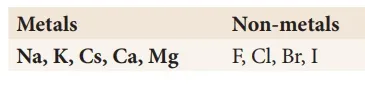
Compound + Element → Compound
In this case, a compound reacts with an element to form a new compound. For instance, phosphorous trichloride reacts with chlorine gas and forms phosphorous pentachloride.
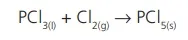
Compound + Compound → Compound
It is a reaction between two compounds to form a new compound. In the following reaction, silicon dioxide reacts with calcium oxide to form calcium silicate.
SiO2(s) + CaO(s) → CaSiO3(s)
Most of the combination reactions are exothermic in nature. Because, they involve the formation of new bonds, which releases a huge amount of energy in the form of heat.
(b) Decomposition reactions
In a decomposition reaction, a single compound splits into two or more simpler substances under suitable conditions. It is the opposite of the combination reaction. The generalised scheme of a decomposition reaction is given below:
Breaking of bonds is the major phenomenon in a decomposition reaction and hence it requires energy to break the bonds, depending on the nature of the energy used in the decomposition reaction.
There are three main classes of decomposition reactions. They are
(i) Thermal Decomposition Reactions
(ii) Electrolytic Decomposition Reactions
(iii) Photo Decomposition Reactions
(i) Thermal Decomposition Reactions
In this type of reaction, the reactant is decomposed by applying heat. For example, on heating mercury (II) oxide is decomposed into mercury metal and oxygen gas. As the molecule is dissociated by the absorption of heat, it is otherwise called ‘Thermolysis’. It is a class of compound to element/element decomposition. i.e. a compound (HgO) is decomposed into two elements (Hg and Oxygen).
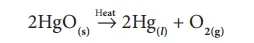
Similarly, when calcium carbonate is heated, it breaks down in to calcium oxide and carbon dioxide. It is a type of compound to compound/compound decomposition.
CaCO3(s) Heat→ CaO(s) + CO2(g)
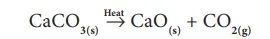
In thermal decomposition reaction, heat is supplied to break the bonds. Such reactions, in which heat is absorbed, are called ‘Endothermic reactions’.
(ii) Electrolytic Decomposition Reactions
In some of the decomposition reactions, electrical energy is used to bring about the reaction. For example, decomposition of sodium chloride occurs on passing electric current through its aqueous solution. Sodium chloride decomposes in to metallic sodium and chlorine gas. This process is termed as ‘Electrolysis’.
2NaCl(aq)→Electricity → 2Na(s) + Cl2(g)

Here, a compound (NaCl) is converted into elements (Na and chlorine). So it is a type of compound to element/element decomposition.
(iii) Photo Decomposition Reactions
Light is an another form of energy, which facilitates some of the decomposition reactions. For example, when silver bromide is exposed to light, it breaks down into silver metal and bromine gas. As the decomposition is caused by light, this kind of reaction is also called ‘Photolysis’.
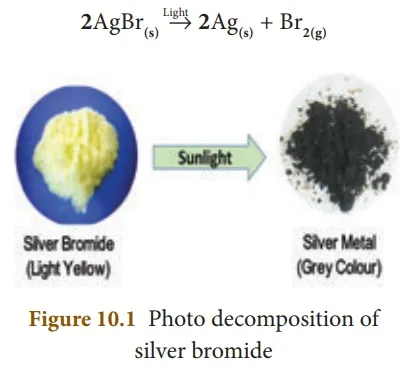
The yellow coloured silver bromide turns into grey coloured silver metal. It is also a compound to element/element decomposition.
(c) Single Displacement Reactions
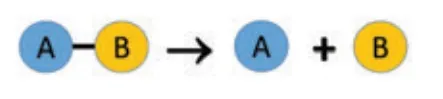
It is a reaction between an element and a compound. When they react, one of the elements of the compound-reactant is replaced by the element-reactant to form a new compound and an element. The general schematic representation of a single displacement reaction is given as:

‘A’ displaces element ‘B’ from the compound ‘BC’ and hence a single displacement reaction occurs. If zinc metal is placed in hydrochloric acid, hydrogen gas is evolved. Here, hydrogen is displaced by zinc metal and zinc chloride is formed.
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
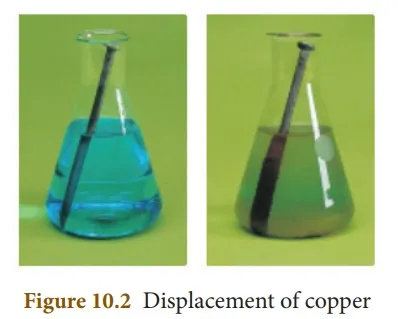
Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
If an iron nail is placed in an aqueous solution of copper (II) sulphate as shown in Fig. 10.2, the iron displaces copper from its aqueous solution and the so formed copper deposits over the iron nail.
It is easy to propose so many reactions of this kind with different combinations of reactants. Will they all occur in practice? No. This is most easily demonstrated with halogens. Let us consider the following two reactions:
2NaCl(aq) + F2(g) → 2NaF(aq) + Cl2(g)
2NaF(aq) + Cl2(g) → 2NaCl(aq) + F2(g)
The first reaction involves the displacement of chlorine from NaCl, by fluorine. In the second reaction, chlorine displaces fluorine from NaF. Out of these two, the second reaction will not occur. Because, fluorine is more active than chlorine and occupies the upper position in the periodic table. So, in displacement reactions, the activity of the elements and their relative position in the periodic table are the key factors to determine the feasibility of the reactions. More active elements readily displace less active elements from their aqueous solution.
The activity series of some elements is given below:
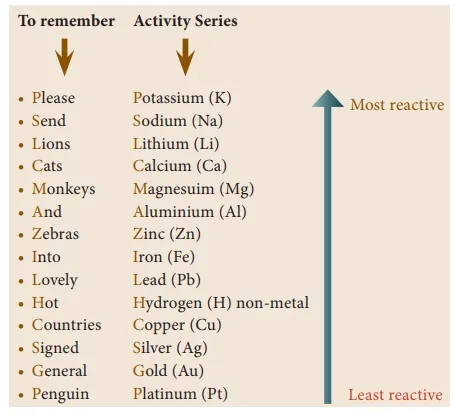
By referring the activity series, try to answer the following questions:
Which of the metals displaces hydrogen gas from hydrochloric acid? Silver or Zinc. Give the chemical equation of the reaction and Justify your answer.
d) Double Displacement Reactions
When two compounds react, if their ions are interchanged, then the reaction is called double displacement reaction. The ion of one compound is replaced by the ion of the another compound. Ions of identical charges are only interchanged, i.e., a cation can be replaced by other cations. This reaction is also called ‘Metathesis Reaction’. The schematic representation of a double displacement reaction is given below:
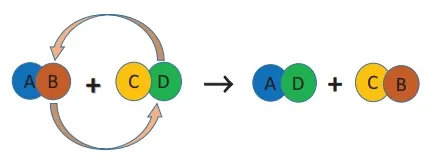
For a double displacement reaction to take place, one of the products must be a precipitate or water. By this way, there are major classes of double displacement reactions. They are:
(i) Precipitation Reactions
(ii) Neutralization Reactions
(i) Precipitation Reactions
When aqueous solutions of two compounds are mixed, if they react to form an insoluble compound and a soluble compound, then it is called precipitation reaction. Because the insoluble compound, formed as one of the products, is a precipitate and hence the reaction is so called.
When the clear aqueous solutions of potassium iodide and lead (II) nitrate are mixed, a double displacement reaction takes place between them.
Pb(NO3)2(aq) + 2KI(aq) → PbI2(s)↓ + 2KNO3(aq)
Potassium and lead displace or replace one other and form a yellow precipitate of lead (II) iodide as shown in Fig. 10.3.

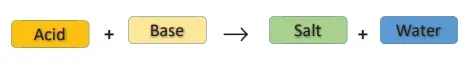
(ii) Neutralization Reactions
In your lower classes, you have learned the reaction between an acid and a base. It is another type of displacement reaction in which the acid reacts with the base to form a salt and water. It is called 'neutralization reaction' as both acid and base neutralize each other.
Reaction of sodium hydroxide with hydrochloric acid is a typical neutralization reaction. Here, sodium replaces hydrogen from hydrochloric acid forming sodium chloride, a neutral soluble salt.
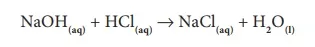
Similarly, when ammonium hydroxide reacts with nitric acid, it forms ammonium nitrate and water.
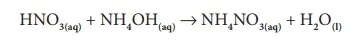
(e) Combustion Reactions
A combustion reaction is one in which the reactant rapidly combines with oxygen to form one or more oxides and energy (heat). So in combustion reactions, one of the reactants must be oxygen. Combustion reactions are majorly used as heat energy sources in many of our day to day activities. For instance, we use LPG gas for domestic cooking purposes. We get heat and flame from LPG gas by its combustion reaction of its constituent gases. LPG is a mixture of hydrocarbon gases like propane, butane, propylene, etc. All these hydrocarbons burn with oxygen to form carbon dioxide and water.
C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g) + Heat
Propane
Since heat is evolved, it is an exothermic reaction. As oxygen is added, it is also an oxidation. So, combustion may be called as an exothermic oxidation. If a flame is formed (as shown in Fig. 10.4), then it is called burning.

Which of the following is a combustion?
(i) Digestion of Food
(ii) Rusting of iron
Many thousands of reactions fall under these five categories and further you will learn in detail about these reactions in your higher classes.