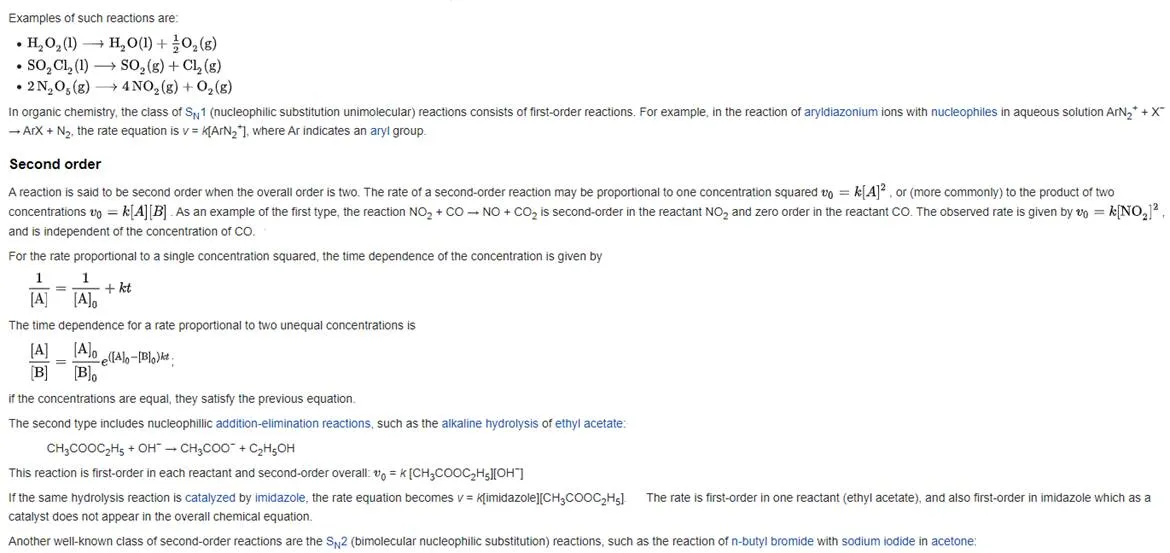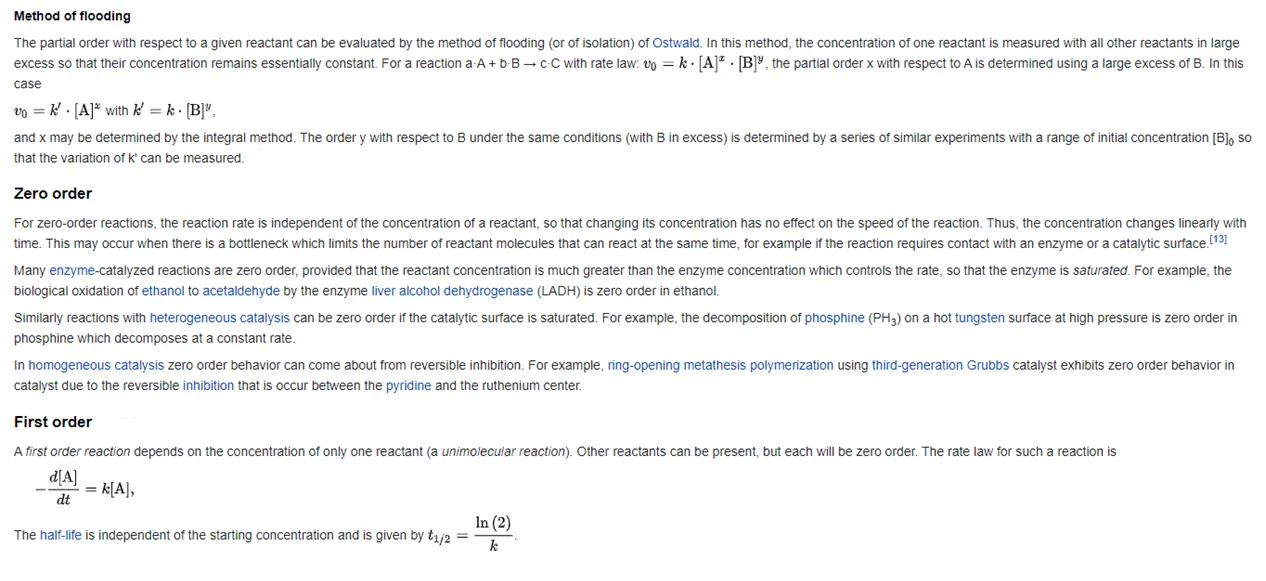
Rate equation
The rate law or rate equation for a chemical reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial reaction orders).[1] For many reactions, the initial rate is given by a power law such as

Definition
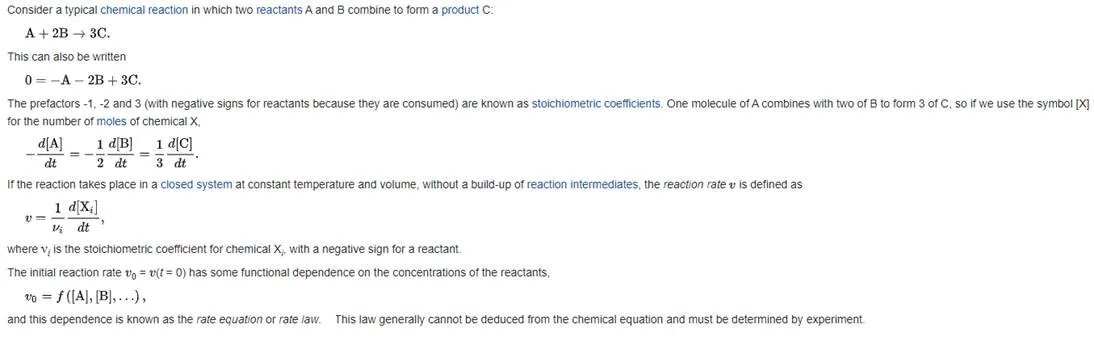
Power laws