Thermodynamics: Difference between Irreversible and Reversible process
Reversible process. In a reversible process the series of changes carried out on the system during its transformation from initial to final state may be possibly reversed in an exact manner.
This is possible when the changes are carried out very slowly in many smaller steps on the system during its change from initial to final state. By doing so, each of its intermediate state will be in equilibrium with its surroundings. Under such conditions the initial and final states of the system become reversible completely.
For example, when ice melts a certain amount of heat is absorbed. The water formed can be converted back to ice if the same amount of heat is removed from it. This indicates that many reversible processes are non-spontaneous processes also.
Irreversible Process
An irreversible process is one which cannot be retraced to the initial state without making a permanent change in the surroundings. Many of the spontaneous processes are irreversible in nature.
For eg. Biological ageing is an irreversible process. Water flowing down a hill on its own accord is an irreversible process.
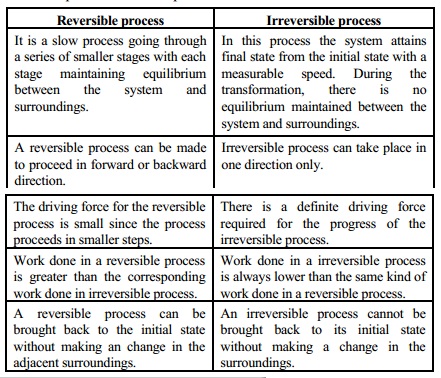
Some of the characteristics of thermodynamically reversible and irreversible processes are compared as below:

Reversible process
1. It is a slow process going through a series of smaller stages with each stage maintaining equilibrium between the system and surroundings.
2. A reversible process can be made to proceed in forward or backward direction.
3. The driving force for the reversible process is small since the process proceeds in smaller steps.
4. Work done in a reversible process is greater than the corresponding work done in irreversible process.
5. A reversible process can be brought back to the initial state without making an change in the adjacent surroundings.
Irreversible process
1. In this process the system attains final state from the initial state with a measurable speed. During the transformation, there is no equilibrium maintained between the system and surroundings.
2. Irreversible process can take place in one direction only.
3. There is a definite driving force required for the progress of the irreversible process.
4. Work done in a irreversible process is always lower than the same kind of work done in a reversible process.
5. An irreversible process cannot be brought back to its initial state without making a change in the surroundings.