Hydration
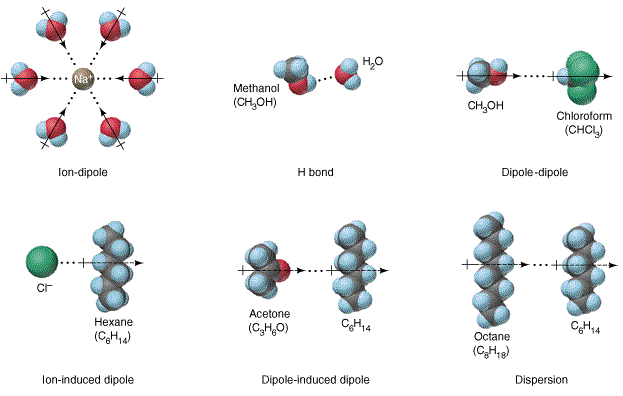
The formation of a solution involves the interaction of solute with solvent molecules. Many different liquids can be used as solvents for liquid solutions, and water is the most commonly used solvent. When water is used as the solvent, the dissolving process is called hydration. The interaction between water molecules and sodium ion is illustrated as one of the diagram below. This is a typical ion-dipole interaction. At the molecular level, the ions interact with water molecules from all directions in a 3-dimensional space. This diagram depicts the concept of interaction only.

Figure 11: Intermolecular interactions.
The above diagram also display hydrogen-bonding, dipole-dipole, ion-induced dipole, and dipole-induced dipole interactions. In the absence of these interactions, solvation takes place due to dispersion. Definitions of these terms are obvious from the diagrams. The meaning of the words used in the term also hints the nature of the interactions.
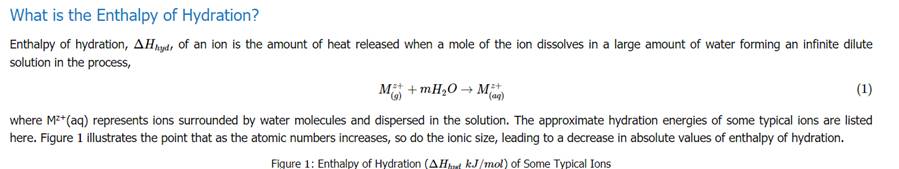
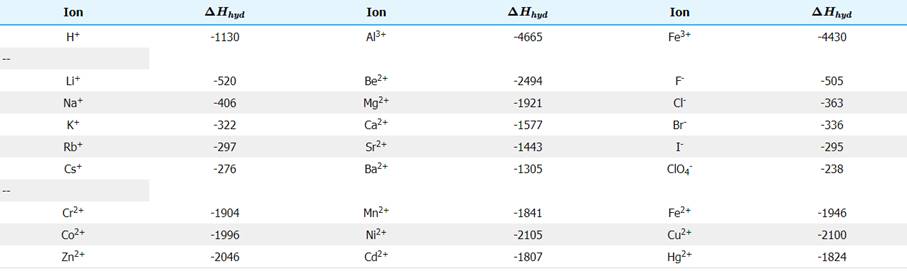
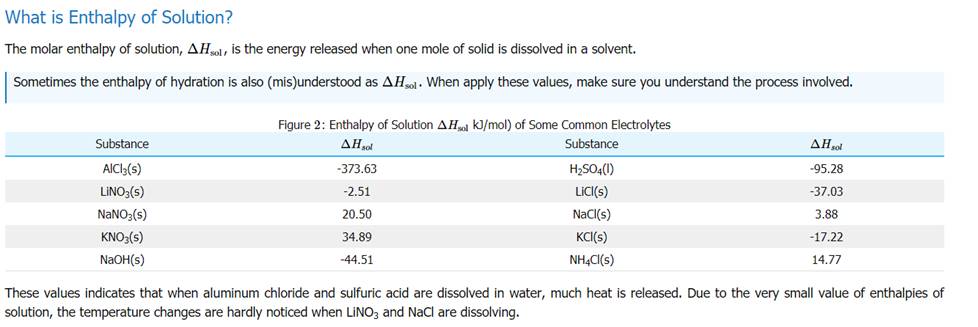
From the above table, an estimate can be made for the hydration energy of sodium chloride. The hydration energy of an ionic compound consists of two inseparable parts. The first part is the energy released when the solvent forms a coordination compound with the ions. This energy released is called the Enthalpy of ligation, ΔHlig. The processes related to these energies are shown below:
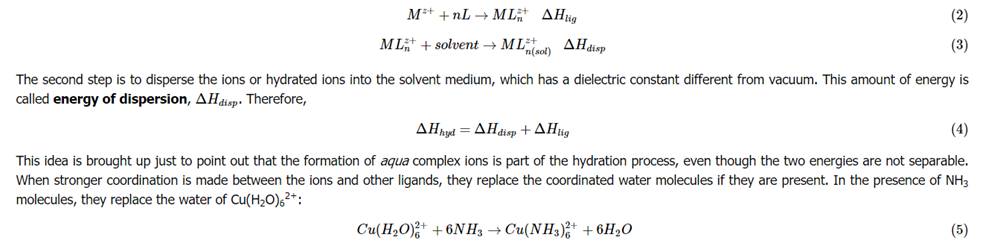
Relating Hydration Energy to Lattice Enthalpy
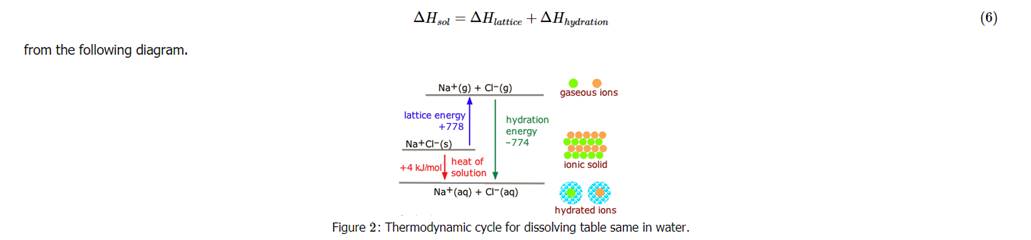
In the discussion of lattice energy, we consider the ions separated into a gas form whereas in the dissolution process, the ions are also separated, but this time into ions dispersed in a medium with solvent molecules between ions. The medium or solvent has a dielectric constant. The molar enthalpy of solution, ΔHsolΔHsol, is the energy released when one mole solid is dissolved in a solvent. This quantity, the enthalpy of crystallization, and energy of hydration forms a cycle. Taking the salt NaClNaCl as an example, the following relationship is obvious,