Heat of Sublimation
The molar heat (or enthalpy) of sublimation is the amount of energy that must be added to a mole of solid at constant pressure to turn it directly into a gas (without passing through the liquid phase). Sublimation requires all the forces are broken between the molecules (or other species, such as ions) in the solid as the solid is converted into a gas. The heat of sublimation is generally expressed as ΔHsubΔHsub in units of Joules per mole or kilogram of substance.
Introduction
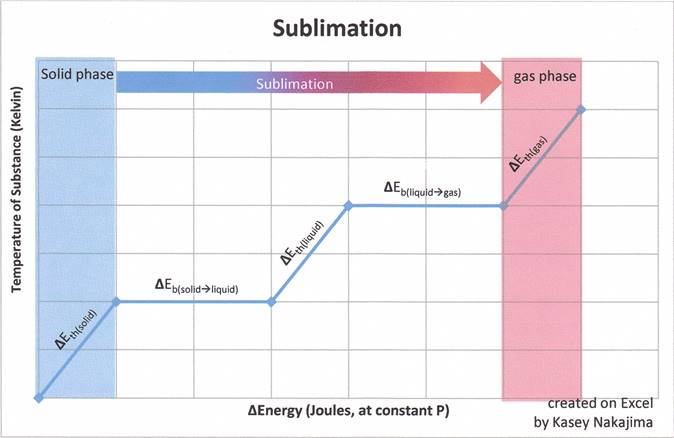
Sublimation is the process of changing a solid into a gas without passing through the liquid phase. To sublime a substance, a certain energy must be transferred to the substance via heat (q) or work (w). The energy needed to sublime a substance is particular to the substance's identity and temperature and must be sufficient to do all of the following:
· Excite the solid substance so that it reaches its maximum heat (energy) capacity (q) in the solid state.
· Sever all the intermolecular interactions holding the solid substance together
· Excite the unbonded atoms of the substance so that it reaches its minimum heat capacity in the gaseous state
Decomposing ΔHsubΔHsub
Although the process of sublimation does not involve a solid evolving through the liquid phase, the fact that enthalpy is a state function allows us to construct a "thermodynamic cycle" and add the various energies associated with the solid, liquid, and gas phases together (e.g., Hess' Law).

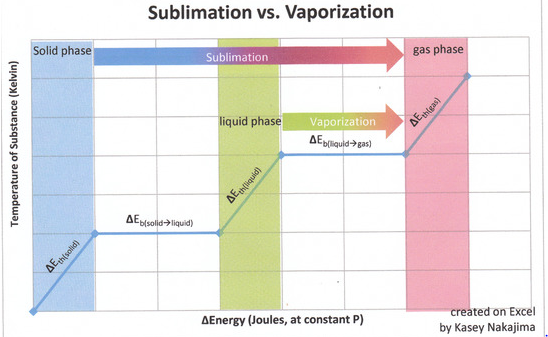
Figure 1: The graph below indicates the inclusion of the liquid phase, the graph is merely a representation of how much energy is needed to sublime a solid substance. Recall that sublimation does not include the liquid phase and that the fact that enthalpy is a state function allows us to add the enthalpies of fusion and vaporization together to find the enthalpy of sublimation.
The energies involved in sublimation can be expressed by the sum of the enthalpy changes for each step:
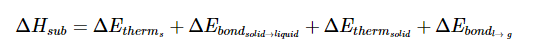
Recall that for state functions, only the initial and final states of the substance are important. Say for example that state A is the initial state and state B is the final state. How a substance goes from state A to state B does not matter so much as what state A and what state B are. Concerning the state function of enthalpy, the energies associated with enthalpies (whose associated states of matter are contiguous to one another) are additive. Though in sublimation a solid does not pass through the liquid phase on its way to the gas phase, it takes the same amount of energy that it would to first melt (fuse) and then vaporize.
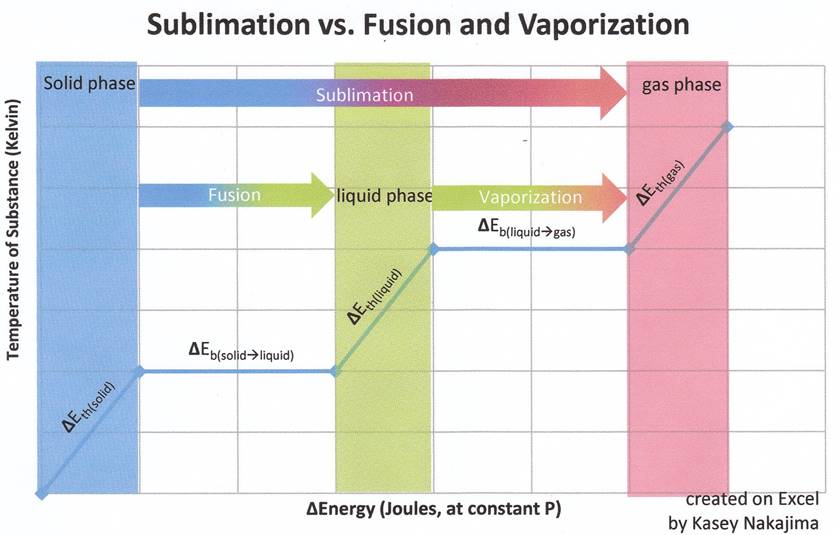
A change in thermal energy is indicated by a change in temperature (in Kelvin) of a substance at any particular state of matter. Change in thermal energy is expressed by the equation