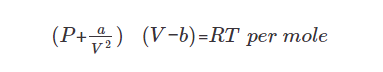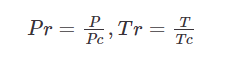
Equation of state for non-ideal gases
PV=ZRT, z : compressibility factor
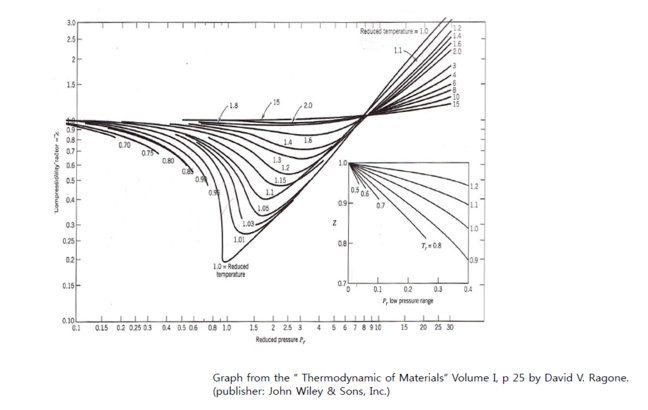
- Z can be estimated using the concept of “corresponding states”.
It states that gases behave similarly with respect to non-ideality;
that is, they have the same Z value when they are in corresponding
states relative to their respective critical pressure and critical
temperature.
- Thus, Z can be determined by knowing reduced pressure Prr, and
reduced temperature Trr.
- The Z values are tabulated (graphed) as a function of Prr, Trr, thus Z
depends on P, T.
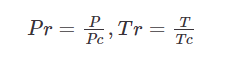
where P, and T are the actual pressure and temperature and Pc
and Tc are the critical values.
2) Van der Waals (vdw) equation of state
From ideal gas law, corrections in
① Volume
Real gas has finite volume (ideal gas = volume-less)
→ subtract the gas volume from the total volume.
② Another consideration
Particles are interacting each other. This correction should be considered in the pressure term.